氙
原子序數為54的化學元素 来自维基百科,自由的百科全书
| 外觀 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
無色氣體,在高壓放電管中呈現紫藍色 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 概況 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 名稱·符號·序數 | 氙(Xenon)·Xe·54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 元素類別 | 稀有氣體 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 族·週期·區 | 18·5·p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 標準原子質量 | 131.293(6)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
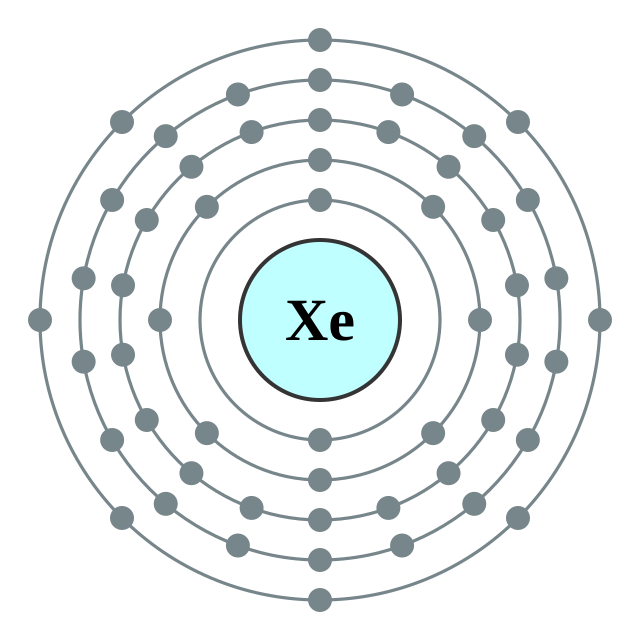
| 電子排布 | [Kr] 4d10 5s2 5p6 2, 8, 18, 18, 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 歷史 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 發現 | 威廉·拉姆齊和莫里斯·特拉弗斯(1898年) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 分離 | 威廉·拉姆齊和莫里斯·特拉弗斯(1898年) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 物理性質 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 物態 | 氣態 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 | (0 °C, 101.325 kPa) 5.894 g/L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 沸點時液體密度 | 3.057[2] g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 熔點 | (101.325 kPa)161.4 K,-111.7 °C,-169.1 °F | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 沸點 | (101.325 kPa)165.03 K,-108.12 °C,-162.62 °F | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 三相點 | 161.405 K(−112 °C),81.6[3] kPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 臨界點 | 289.77 K,5.841 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 熔化熱 | (101.325 kPa)2.27 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 汽化熱 | (101.325 kPa)12.64 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 比熱容 | 5R/2 = 20.786 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
蒸氣壓
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 原子性質 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 氧化態 | 0, +1, +2, +4, +6, +8 (很少大於0) (氧化物具弱酸性) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 電負性 | 2.6(鮑林標度) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 電離能 | 第一:1170.4 kJ·mol−1 第二:2046.4 kJ·mol−1 第三:3099.4 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 共價半徑 | 140±9 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 范德華半徑 | 216 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 雜項 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 晶體結構 | 面心立方 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 磁序 | 抗磁性[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 熱導率 | 5.65×10-3 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 聲速 | (液態)1090 m/s;(氣態)169 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS號 | 7440-63-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 同位素 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
主條目:氙的同位素
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
自然產生的氙由7種穩定同位素組成。氙還有40多種不穩定的放射性同位素。氙同位素的相對比例對研究太陽系早期歷史有重要的作用。[17]具放射性的氙-135是核反應爐中最重要的中子吸收劑,可通過碘-135發生負β衰變產生。[18]
氙可用在閃光燈[19]和弧燈中,[20]或作全身麻醉藥。[21]最早的準分子激光設計以氙的二聚體分子(Xe2)作為激光介質,[22]而早期激光設計亦用氙閃光燈作激光抽運。[23]氙還可以用來尋找大質量弱相互作用粒子[24],或作航天器離子推力器的推進劑。[25]
歷史
英國化學家威廉·拉姆齊和莫里斯·特拉弗斯(Morris Travers)在發現了氪和氖後,於1898年7月12日在蒸發液態空氣後的殘留物中發現了氙。[26][27]拉姆齊建議把這一新元素命名為「xenon」,源自希臘語「ξένον」(xenon),即「ξένος」(xenos)的中性單數形,意為外來者、陌生人或異客。[28][29]1902年,拉姆齊估算氙在地球大氣中的含量為2千萬分之一。[30]
1930年代,美國工程師哈羅德·尤金·艾傑頓(Harold Eugene Edgerton)開始為高速攝影研究頻閃燈,並發明了氙閃光燈。在氙閃光燈中,電流短暫通過含有氙氣的玻璃管,使其發光。到了1934年,艾傑頓已經能夠產生1微秒長的閃光。[19][31][32]
1939年,美國醫生阿爾伯特·本克(Albert R. Behnke Jr.)著手研究深海潛水員有「酒醉感」的原因。他在測試對象所呼吸的氣體中調整各種氣體的比例,並發現潛水員對深度的感覺有所變化。他以此推論,氙氣能夠用於麻醉。俄羅斯毒理學家尼克拉·拉薩列夫(Nikolay V. Lazarev)曾在1941年研究過氙麻醉藥,但直到1946年美國醫學家約翰·勞倫斯(John H. Lawrence)才發表了他對老鼠進行的一項實驗研究,首次證實了氙作為麻醉藥的效用。1951年,美國麻醉師斯圖爾特·科林(Stuart C. Cullen)第一次使用氙麻醉藥,並成功為兩名病人進行了手術。[33]
氙以及其他稀有氣體曾一直被認為是完全惰性的,無法形成化合物。不過,化學家尼爾·巴特萊特(Neil Bartlett)在不列顛哥倫比亞大學任教時,發現六氟化鉑(PtF6)氣體是一種強氧化劑,能夠氧化氧氣(O2),形成六氟合鉑酸氧(O2+[PtF6]–)。[34]因為O2和氙的第一電離能幾乎相同,所以巴特萊特猜想,氙也有可能可以被六氟化鉑氧化。1962年3月23日,他將這兩種氣體混合,產生了第一種稀有氣體化合物六氟合鉑酸氙。[35][16]他當時認為該氣體產物為Xe+[PtF6]–,但之後的分析表明該氣體很可能是多種氙鹽的混合物。[36][37][38]此後,許多其他的氙化合物也陸續被發現,[39]而同時被發現的還包括氬、氪和氡等稀有氣體的化合物,如氟氬化氫(HArF)、[40]二氟化氪(KrF2)[41][42]及二氟化氡。[43]到了1971年,已知的氙化合物已經超過了80種。[44][45]
性質

氙的原子序為54,即每一個氙原子核中共有54顆質子。在標準溫度和壓力下,純氙氣的密度為5.761 kg/m3,也就是地球地面大氣密度(1.217 kg/m3)的4.5倍左右。[46]當處於液態時,氙的密度可高達3.100 g/mL,最高密度在三相點處達到。[47]固態氙的密度為3.640 g/cm3,比花崗岩的2.75 g/cm3更高。[47]當壓力超過10億帕斯卡時,氙會呈金屬態。[48]
在大約140 GPa壓力下,固體氙的晶體結構會從面心立方轉變為六方密排,並開始呈現金屬特性。氙在155 GPa壓力以上完全進入金屬態。這時候的氙會吸收紅光,因此會呈天藍色。這一特性在金屬中較為罕見,原因是氙在金屬態下的電子能帶寬度較小。[49][50]
氙的化合價為0,與其他零價元素同屬於稀有氣體,亦稱惰性氣體。氙對大部份化學反應都呈惰性(如燃燒反應),因為它有8個價電子。這使外層電子處於最低能量組態,因此非常穩定。[51]
當電弧通過裝有氙氣的玻璃管時,氙會被激發而發出藍至淡紫色光。氙的發射譜線橫跨整個可見光譜,[52]其最強的光譜線位於藍光部份,所以整體發藍光。[53]
存量及生產
氙是地球大氣層中的一種微量氣體,含量約為10億分之87±1(nL/L),亦即1150萬分之一。[54]某些天然礦泉也會釋放出含有氙的氣體。[55][56]
氙是空氣氮氧分離過程的副產品。這一過程一般在雙柱式分餾塔中進行,所產生的液氧中會含有少量的氪和氙。再進行更多的分餾步驟之後,液氧中的氪和氙含量可以提高至0.1至0.2%。這些氪和氙可以通過硅膠吸附或蒸餾提取出來,混合物再經蒸餾分離成氪和氙。[57][58]從大氣層中提取一升氙氣需要220瓦小時的能量。[59]1998年,全球氙產量為5千至7千 m3。[60]由於含量稀少,氙的價格比其他更輕的稀有氣體高許多:1999年歐洲的氙氣價格為每升10歐元,氪氣每升1歐元,氖氣每升0.20歐元,[60]
在太陽系以內,氙元素的核素比例為1.56 × 10−8,質量豐度為63萬分之一。[61]太陽大氣層、地球、小行星及彗星中的氙含量很低。木星大氣層含有異常高的氙元素,含量約為太陽的2.6倍。[62][注 1]這一現象的原因不詳,但有可能是因為在太陽系形成早期太陽星雲溫度提升之前,微行星迅速堆積所致。[63]地球上的氙存量很低,這可能是由於氙和氧在石英中產生共價鍵,從而減少氙被釋入大氣層的量。[64]
與輕稀有氣體不同的是,恆星核合成過程無法製造氙元素。所有包括氙在內比鐵-56更重的元素經核聚變合成時,會產生凈能量損失,因此無法在恆星內部形成。[65]能夠形成氙的自然過程包括:超新星爆炸,[66]紅巨星用盡氫燃料進入漸近巨星分支後的慢中子捕獲過程(s-過程),[67]一般新星爆炸,[68]以及碘、鈾和鈈等元素的放射性衰變。 [69]
同位素
自然形成的氙共由7種穩定同位素組成,在各元素中排第二位。第一位是錫,其穩定同位素共有10個。穩定同位素數量高於7個的元素只有錫。[70]126Xe和134Xe理論上能雙重β衰變,但這未經實驗證明,因此該同位素仍被認為是穩定的。[71]除這些穩定同位素之外,氙還有40多種不穩定同位素。其中壽命最長的為124Xe,它會進行雙電子俘獲衰變,半衰期為1.8×1022年。[7]129I在β衰變後,會產生129Xe同位素。該反應的半衰期為1600萬年。另外131mXe、133Xe、133mXe和135Xe都是235U和239Pu的核裂變產物,[69]因此被用作探測核爆炸的發生。[72]
氙的其中兩種穩定同位素129Xe和131Xe具有非零的固有角動量(自旋,可用於核磁共振)。利用圓極化光和銣氣體,氙的核自旋對齊可以超越普通的極化。[73]如此產生的自旋極化能夠超過其最高可能值的50%,遠遠大於玻爾茲曼分佈的平衡值(在室溫下通常不超過最高值的0.001%)。這種非平衡態的自旋對齊是短暫的,稱為超極化現象。對氙進行超極化的過程叫做光抽運(但不同於激光抽運)。[74]
由於129Xe原子核的自旋為1/2,所以其電四極矩為零,故129Xe核在與其他原子撞擊時,不會有任何四極相互作用。這使得它的超極化狀態能夠持續更長的時間,甚至在激光束關閉及鹼氣體在室溫表面冷凝後,仍能保留該狀態。129Xe的自旋極化在血液中能持續數秒,[75]在氣態下持續數小時,[76]並在深度冷凍的固態下持續數天。[77]相比之下,131Xe的核自旋為3/2,四極矩不為零,其T1弛豫時間位於毫秒至秒區間內。[78]
氙的某些同位素,如133Xe和135Xe,可在核反應爐中對可以裂變物質進行中子照射產生。[14]135Xe在核裂變反應爐中具有重要的作用。135Xe的熱中子截面很高(2.6×106靶恩),[18]因此可用作中子吸收劑或中子毒物,從而減慢或停止連鎖反應。美國曼哈頓計劃中用來產生鈈元素的最早期反應爐就用到了氙的這一作用。[79]135Xe在反應爐中作為中子毒物,對車諾比核災有著重要的影響。[80]反應爐的關閉或功率的降低可以造成135Xe的積聚,使反應爐進入所謂的氙中毒狀態(又稱氙坑、碘坑)。[81][82]
在不利條件下,高濃度的放射性氙同位素可以從核反應爐中釋放出來,來源包括裂變產物從開裂的燃料棒中釋出,[83]或冷卻水中的鈾進行裂變。[84]
隕石中的氙同位素比例可以用來研究太陽系的形成和演化。碘氙放射性定年法可以測定核合成至太陽星雲中固體物體縮合之間的時間。1960年,物理學家約翰·雷諾(John H. Reynolds)發現某些隕石中的氙-129含量異常高。他推斷這是碘-129的衰變產物。這一同位素可經宇宙射線散裂和核裂變緩慢產生,但只有在超新星爆炸中才能大量產生。由於129I的半衰期(1600萬年)相對宇宙時長來說非常短,因此可推論從超新星爆炸到隕石凝固之間經過的時間很短。一顆超新星在太陽系形成前不久爆炸,產生129I同位素之餘,可能也導致了前太陽氣體雲的收縮。[85][86]
利用類似的方法,其他氙同位素比例也可以用來研究行星分化和氣體釋放過程,包括129Xe/130Xe和136Xe/130Xe。[17]例如,火星大氣層的氙含量與地球相似,約為百萬分之0.08,[87]但其129Xe比例比地球和太陽高。這一同位素是由放射性衰變產生的,所以火星很可能在形成後約1億年以內喪失了大部份的原始大氣。[88][89]美國新墨西哥州二氧化碳井氣中所發現的高比例129Xe是地球形成不久後經地幔核衰變產生的氣體之一。[69][90]
化合物
尼爾·巴特萊特在1962年發現氙能夠形成化合物之後,許多其他的氙化合物也陸續被發現和研究。幾乎所有氙化合物都含有電負性高的氟或者氧。[91]


氙共有三種已知氟化物:二氟化氙(XeF
2)、四氟化氙(XeF
4)及六氟化氙(XeF
6)。理論預測XeF是不穩定的。[92]它們是合成其它氙化合物的起點。
二氟化氙(XeF
2)是一種固體晶體,在氟與氙混合物經紫外光照射後形成,[93](使用一般日光含的紫外光就已足夠。)[94]XeF
2在高溫下用NiF
2催化劑長時間加溫會產生XeF
6。[95]XeF
6在NaF中經熱裂解後可以形成高純度XeF
4。[96]
氙的氟化物都可以作為氟離子受體和予體,形成如XeF+
和Xe
2F+
3等正離子,以及XeF−
5、XeF−
7和XeF2−
8等負離子。XeF
2經氙氣還原後,會形成綠色的順磁性Xe+
2離子。[91]
XeF
2可以與過渡金屬離子形成配合物。已知的配合物超過30種。[95]
雖然人們對氙的氟化物已有一定的了解,但是對其他的鹵化物則幾乎一無所知。二氯化氙(XeCl2)可通過高頻照射氙、氟及四氯化硅或四氯化碳的混合物而成,[97]據稱是一種吸熱的無色晶體,在80°C以上分解成其組成元素。但是人們未知XeCl
2是確實的化合物,還是由氙原子和Cl
2分子弱結合形成的范德華分子。[98]理論計算指出,直線型XeCl
2分子比范德華分子較不穩定。[99]更不穩定的四氯化氙則不能通過化學方法合成,只能通過放射性的129
ICl−
4衰變產生。[100][101]
氙共有三種已知氧化物:二氧化氙(XeO
2)、三氧化氙(XeO
3)及四氧化氙(XeO
4)。二氧化氙在2011年被發現,配位數為4,[102]XeO2是在四氟化氙與水冰反應後形成的。其晶體結構特殊,有可能能夠取代硅酸鹽礦物中的硅。[103]科學家利用紅外光譜分析在固體氬當中發現了XeOO+正離子。[104]固態三氧化氙和四氧化氙都是爆炸性很強的物質。[105][106]
氙不會和氧直接進行反應。三氧化氙是經XeF
6的水解反應形成的:[107]
XeO
3具弱酸性,會在鹼中溶解成含HXeO−
4負離子的不穩定氙酸鹽。這些不穩定鹽會很快歧化成氙氣和含XeO4−
6負離子的高氙酸鹽。[108]
高氙酸鈉或高氙酸鋇在濃硫酸中會產生淺黃色固態四氧化氙:[97]
氙有多個已知的氟氧化物,包括二氟一氧化氙(XeOF
2)、四氟一氧化氙(XeOF
4)、二氟二氧化氙(XeO
2F
2)及二氟三氧化氙(XeO
3F
2)。二氟化氧(OF
2)與氙氣在低溫下反應會形成XeOF
2;XeF
4的部份水解也可產生XeOF
2。它在−20℃以上會歧化成XeF
2和XeO
2F
2。[109]XeF
6的部份水解會產生XeOF
4[110];XeF
6與高氙酸鈉(Na
4XeO
6)反應後也可形成XeOF
4。第二種反應會同時產生少量的XeO
3F
2。XeOF
4與CsF反應後會形成XeOF−
5負離子,[109][111]而XeOF3會和KF、RbF和CsF反應形成XeOF−
4負離子。[112]
氙的氟化物都與水發生反應,XeF2在水中的溶解度為0.15 mol/L(273K),能將水氧化。
XeF4與水的反應中,一半用於氧化水,另外一半發生了歧化反應。總反應為:
XeF6有強氧化性,但由於生成的XeO3在水中溶解且穩定,XeF6在水中只發生水解反應。
氙可以與電負性比氟和氧低的元素形成化合物,特別是碳元素。[113]要使這些化合物穩定,必須使用吸電子基團,如經氟取代形成的基團。[108]已知的含碳化合物包括:[109][114]
- C
6F
5–Xe+
–N≡C–CH
3,其中的C6F5是五氟苯基。 - [C
6F
5]
2Xe - C
6F
5–Xe–X,X可以是CN、F或Cl。 - R–C≡C–Xe+
,R可以是C
2F−
5或叔丁基。 - C
6F
5–XeF+
2 - (C
6F
5Xe)
2Cl+
其他含有電負性較低的氙化合物包括F–Xe–N(SO
2F)
2和F–Xe–BF
2。F–Xe–BF
2可以從四氟硼酸二氧O
2BF
4在−100℃溫度下合成。[109][115]
四氙合金(II)離子(AuXe2+
4)非常特殊,它含有氙﹣金鍵。[116]氙和金都是極不活躍的元素,成鍵時以氙作為過渡金屬配位體。之一離子出現在AuXe
4(Sb
2F
11)
2當中。
Xe
2Sb
2F
11含有氙﹣氙鍵,這是兩個元素間已知最長的鍵(308.71 pm = 3.0871 Å)。[117]
1995年,芬蘭赫爾辛基大學的馬爾庫·拉薩寧(Markku Räsänen)等人宣佈成功合成HXeH,並其後宣佈合成HXeOH、HXeCCH以及其他氙化合物分子。[118]2008年,利奧尼德·赫里亞切夫(Leonid Khriachtchev)等人宣佈,他們在低溫氙基體內對水進行光解後合成了HXeOXeH。[119]他們也合成了含氘的分子,如HXeOD和DXeOH。[120]
除了可以在化合物中形成化學鍵之外,氙原子還能嵌在另一種化合物的晶體結構當中,形成包合物。這包括水合氙(Xe·5.75 H2O),其中氙原子位於水分子形成的晶體結構空隙中。[121]這種包合物的熔點為24℃。[122]科學家也改用氘合成了該包合物。[123]這類水合包合物可以在高壓條件下自然形成,例如南極洲冰蓋下的沃斯托克湖。[124]包合物的形成可以用在分餾過程中,以分離氙、氬和氪。[125]
氙還可以形成內嵌富勒烯化合物,即內嵌氙原子的富勒烯分子。富勒烯裡面的氙原子可以通過129Xe核磁共振光譜分析來觀測。科學家可利用這種方法分析富勒烯分子的化學反應,因為其中的氙原子對周圍環境十分敏感,並進行化學位移。然而,氙原子也會對富勒烯的化學活性產生影響。[126]
當氙原子處於基態的時候,會互相排斥,無法成鍵。但當它們受到激發後,就能夠形成準分子(激發態二聚體),直到電子回到基態。氙原子一般會填滿其最外電子層,鄰近的氙原子就可以為其提供電子。氙準分子的一般存留時長為1至5納秒,其衰變會釋放波長約為150和173納米的光子。[127][128]氙還能與其他元素結合成準分子,例如溴、氯及氟。[129]
應用
氙可用於發光,應用包括:用於攝影的氙閃光燈,[19]激發激光媒介以產生相干光,以及[130]殺菌燈等。[131]1960年發明的首個固態激光器[23]及推動慣性約束聚變的激光器都用到了氙閃光燈作激光抽運。[132]



氙弧燈能夠連續發光,其色溫近似於正午的日光,因此被用於模擬陽光。1940年氙弧燈進入市場後,開始淘汰壽命較短的碳弧燈作為電影放映機的光源。[20]這種光源被用在一般35毫米膠片、IMAX和新型數碼投影機的電影投影、高強度氣體放電燈車頭燈、高端軍用電筒以及其他專業用途。這種弧燈能發出短波長紫外線,以及可被用於夜視鏡的近紅外線。
等離子顯示器中的發光體裝有氙和氖,並經電極轉化成等離子狀態。該等離子體與電極之間的作用會產生紫外光,從而激發顯示器前部的磷質塗層,發出可見光。[133][134]
氙也被用於啟動高壓鈉燈。氙的熱導率和電離能是所有非放射性稀有氣體中最低的。其化學惰性能避免對化學反應的干預;低熱導率可降低燈在運作時的熱能損失;低電離能則使氙在非高溫狀態下的擊穿電壓相對較低,令燈更容易啟動。[135]
1962年,貝爾實驗室研究人員發現了氙的激光作用,[136]又接著發現在激光介質中加入氦能夠提升激光增益。[137][138]首個準分子激光使用電子來激發氙的二聚體(Xe2),以產生波長為176納米的紫外光,該過程稱為受激發射。[22]氯化氙和氟化氙準分子也可用於激光器中。[139]例如,皮膚病學就用到氯化氙準分子激光。[140]
氙是一種全身麻醉劑。氙較為昂貴,但由於回收循環技術的提升和成本的降低,使用氙的麻醉機將在不久後進入歐洲市場。[59][141]
氙會和多種不同受體和離子通道相互作用。根據理論,這種多模態吸入性麻醉劑很可能具互補性。氙是一種具高親和力的甘氨酸結合部位NMDA受體拮抗劑。[142]不過,與其他的NMDA受體拮抗劑不同的是,氙不具神經毒性,且能夠抑制氯胺酮和一氧化二氮的神經毒性。[143][144]氙不會像氯胺酮和一氧化二氮一樣刺激伏隔核釋放多巴胺。[145]
氙是血清素5-HT3的競爭性抑製劑。這並不產生麻醉或鎮痛的效果,但可以減少麻醉劑相關的噁心和嘔吐感。[146]
氙在40歲人體內的最小肺泡濃度為72%,所以麻醉效果比N2O強44%。[147]因此相對氧氣的使用濃度無需太高,有助避免缺氧。另外與一氧化二氮不同的是,氙不是溫室氣體,所以較為環保。[148]
放射性同位素133Xe的伽馬射線可用來對心、肺和腦進行成像,例如單光子發射電腦攝影。133Xe也被用於測量血流。[149][150][151]
氙是一種很好的磁共振成像(MRI)造影劑。氙氣可以用來對多孔組織的空間和肺泡進行成像。超極化的129Xe同位素在磁共振成像儀中更易檢測,所以被用於研究包括肺在內的各種器官,例如肺內氣體的流動。[152][153]氙可溶於水,又可溶於疏水性溶劑,這有助於對軟組織進行成像。[154][155][156]
由於氙擁有較大、較敏感的外電子層,所以其核磁共振光譜會對氙原子周圍的化學條件有相應的變化。例如,溶於水、疏水性溶劑和某些蛋白質的氙可通過核磁共振波譜法區分開來。[157][158]
氙也應用在表面科學中。核磁共振一般很難檢測樣本的表面,因為表面底下的大量原子核會完全蓋過有用的信號。超極化的氙氣能夠將自身的自旋只傳遞到固體表面,使表面所發出的信號可以與樣本內部的信號區分開來。[159][160]
原子核物理學的氣泡室可以使用氙。[161]氙也可用於任何需要高分子(原子)質量、低反應性物質的用途。核武器試驗所產生的副產品中有具放射性的氙-133和氙-135。通過測量這些同位素,人們可以判斷是否有國家進行核試驗,[162]其中包括朝鮮。[163]

科學家利用液態氙熱量計[164]來測量伽馬射線,並用液態氙尋找大質量弱相互作用粒子(WIMP)。理論預測,當WIMP撞擊氙原子核,會移除一顆電子,產生閃爍。如果使用氙,這一閃爍可以輕易地從其他由宇宙射線所造成的能量爆發分辨開來。[24]不過,意大利大薩索國家實驗室(Laboratori Nazionali del Gran Sasso)的「XENON」實驗以及英國伯比地底實驗室(Boulby Underground Laboratory)的ZEPLIN-II和ZEPLIN-III實驗都還沒有找到證實WIMP存在的證據。雖然沒有發現WIMP,但這些實驗有助於縮小暗物質的可能屬性範圍,以及改進相關的物理模型。[165][166]
氙的電離能很低,是一種很好的航天器離子推力器推進劑。氙在室溫下能夠以液態儲存,在推力器運作時可輕易轉化為氣體。由於氙的化學惰性,它不會對環境造成破壞,或像汞或銫等其他燃料一樣侵蝕離子推進器。1970年代,某些人造衛星開始使用氙離子推進器。[167]美國的深空一號和曙光號探測器以及歐洲的SMART-1飛行器都用到了氙離子推進器。[25][168]
高氙酸鹽可在分析化學中用作氧化劑。二氟化氙是一種硅的腐蝕劑,應用在微機電系統中。[169]二氟化氙與尿嘧啶反應後,會產生抗癌藥物5-氟尿嘧啶。[170]氙在X射線晶體學中可用來研究蛋白質的結構和功用。氙氣在壓力為0.5至5 MPa(5至50 atm)的時候,其原子會結合到蛋白質晶體的疏水性孔穴中。這一產物含有更高質量的原子,但不改變原先的晶體結構,因此可被用於解相位問題。[171][172]
安全
許多含氧的氙化合物都是具有毒性的強氧化劑。同時因為很容易分解成氙元素和氧分子(O2),這些化合物還具有爆炸性。[173]
氙氣在標準溫度和壓力下可以安全地存放在一般的玻璃或金屬容器中。由於氙可溶於大部份塑料和橡膠,因此會從這些材料的容器中慢慢逃逸出去。[174]氙本身並不具毒性,但它可溶於血,並且可以穿透血腦屏障。氙與氧氣混合後吸入,可以達到手術麻醉劑的效果。[173]
氙氣中的音速為每秒169米,比空氣中的音速低。[注 2]這是由於氙原子較氧和氮分子重,因此平均速度較低。當聲道中充滿氙氣時,共振頻率會降低。因此吸入氙氣後說話的音色會比正常低沉,與吸入氦氣後音色提高的現象相反。氙的麻醉效果比一氧化二氮強,而過量吸入氙氣也會造成窒息。因此,許多大學在進行有關氣體改變音色的化學演示時,已不再使用氙氣,而改用分子量相近的六氟化硫氣體。雖然過量吸入六氟化硫仍會造成窒息,但是它不具麻醉效果。[176]
如果氙氣與氧氣混合,而氧氣含量至少有20%,那人體是可以安全吸入的。80%氙氣和20%氧氣的混合氣體會迅速使人失去意識,因此在醫學手術中被用作全身麻醉劑。呼吸作用會有效地混合不同密度的氣體,所以較重的氙氣並不會積聚在肺的底部,而是會和其他氣體一起呼出。[177]然而如果大量氙氣在密閉空間中洩漏出來,會在底部積聚。由於氙無色、無味,所以當人員進入該空間時,很可能會不經意地吸入大量的氙氣。一般的氙氣儲存量並不足以導致這種情況的發生,但在任何缺乏通風的空間中存放氙都具有以上的潛在危險。[178]
備註
參考資料
外部連結
Wikiwand - on
Seamless Wikipedia browsing. On steroids.