热门问题
时间线
聊天
视角
锔
原子序数为96的化学元素 来自维基百科,自由的百科全书
Remove ads
锔是一种银白色的坚硬高密度金属,熔点和沸点是锕系元素中较高的。锔在标准温度和压力下具顺磁性,并在冷却后变为反铁磁性;许多锔化合物也具有磁性的转变。锔在化合物中的氧化态通常为+3和+4,而在溶液中主要呈+3态。锔很容易被氧化,而形成的氧化物是锔最常见的形态。锔可以和各种有机化合物形成萤光配合物,但不出现在任何细菌或古菌中。当摄入人体之后,锔会累积在骨骼、肺部和肝脏中,并可致癌。
锔的所有已知同位素都具有放射性,并具有较小的临界质量(维持核链反应所需的最低质量)。这些同位素主要放射α粒子,辐射释放的热量可以在放射性同位素热电机中用来产生电力。然而由于量的稀少,以及制造费用的昂贵,锔难以用来发电。锔被用于制造更重的锕系元素,及在心律调节器中作为能源的238Pu。它也作为α粒子射源,被用在α粒子X射线光谱仪中。许多火星探测任务都使用该光谱仪来分析火星表面岩石的结构和成分,罗塞塔号的菲莱登陆器(Philae Lander)也用它来探测楚留莫夫-格拉希门克彗星的表面。
Remove ads
历史


虽然过去的核反应实验中很可能已经产生了锔元素,但是要直到1944年,伯克利加州大学的格伦·西奥多·西博格、拉尔夫·A·詹姆斯(Ralph A. James)和阿伯特·吉奥索等人才首次专门合成并分离出锔。他们的实验使用了1.5米直径回旋加速器。[7]
锔的化学辨认是在芝加哥大学的冶金实验室(现阿贡国家实验室)进行的。它是第三个被发现的超铀元素,但在元素周期表中却是第四个超铀元素(当时仍未发现镅)。[8][9]
锔的合成过程如下:首先将硝酸钚溶液涂在面积约为0.5 cm2的铂薄片上,蒸发后的残留物经退火转换为二氧化钚(PuO2)。二氧化钚在回旋加速器中受照射之后,产物溶于硝酸中,再用浓氨水沉淀为氢氧化物。沉淀物溶于高氯酸,再用离子交换分离出锔的某个同位素。由于锔的分离过程十分繁复,以致发现团队最初称其为Pandemonium(希腊文中意为“群魔殿”或“地狱”)。[10][11][12]
1944年7至8月[来源请求],239Pu经α粒子撞击后,产生了锔-242同位素,并释放了一颗中子:[8]
科学家根据衰变时释放的α粒子的特征能量,确切地辨认了锔-242:[8]
这条α衰变的半衰期最初测得为150天[13],后改为162.8天。[14]
1945年3月进行的另一条反应又产生了240Cm同位素:
该同位素的α衰变半衰期最初测得为26.8天[8],后改为30.4天。[14]
锔和镅在1944年的发现与当时旨在制造原子弹的曼哈顿计划息息相关。有关其发现的信息一直保密到1945年才公诸于世。在1945年11月11日美国化学学会正式发布锔和镅的发现前5天,美国电台节目“Quiz Kids”(小朋友问答)的一位听众问到,战时除了镎和钚之外还有没有发现其他新的超铀元素,格伦·西博格回应时泄露了有关发现锔和镅的消息。[10]
锔是以玛丽·居里(Marie Curie)和其丈夫皮埃尔·居里(Pierre Curie)命名的。两人发现了镭元素,并对放射性作出了相当的贡献。这种命名方法参照了元素周期表中位于锔以上的镧系元素钆:钆是以研究稀土元素的科学家、工程师约翰·加多林命名的。[15]
最初制成的锔样本数量很少,肉眼仅仅可见。科学家利用其放射特性,辨认出锔元素。Louis Werner和Isadore Perlman在1947年于加州大学对镅-241进行中子撞击,首次制备了重30 µg的可观量氢氧化锔-242。[16][17][18] W. W. T. Crane、J. C. Wallmann和B. B. Cunningham在1950年制成了宏观量的氟化锔,其磁性和GdF3相似。这首次提供了实验证据,证明锔在其化合物中具+3氧化态。[16]1951年,科学家用钡还原氟化锔,唯一一次制成了金属态的锔。[19][20]
Remove ads
特性


锔是一种放射性人工合成元素,也是质地坚硬、密度高的银白色金属。其物理和化学特性与其上方的镧系元素钆相似。锔的熔点为1340 °C,这比前面的超铀元素镎(637 °C)、钚(639 °C)和镅(1173 °C)都要高,而钆的熔点则在1312 °C。锔的沸点为3110 °C,密度为13.52 g/cm3。这比镎(20.45 g/cm3)和钚(19.8 g/cm3)的密度低,但仍比大部分金属高。锔的两种晶体结构中,α型在标准温度和压力下更稳定。其具有六方对称结构,空间群为P63/mmc,晶格参数a = 365 pm,c = 1182 pm,且每晶胞含四个化学式单位。[21]该晶体具有双六方密排结构,层序为ABAC,并和α镧同型。在23 GPa压力以上及室温下,α锔会转变为β锔。β型具有面心立方对称结构,空间群为Fm3m,晶格常数a = 493 pm。[21]进一步加压到43 GPa后,锔会变为属于正交晶系的γ锔结构,与α铀同型,并一直到52 GPa都不会再有相变。这三种锔的相态也被称为Cm I、II和III。[22][23]
锔的磁特性奇特。其旁边的镅元素在不同温度下都不会偏离居里外斯顺磁性,但α锔会在冷却至65至52 K时转变为反铁磁性,[24][25]而β锔在大约205 K时转变成亚铁磁性。另外,锔和氮族元素的化合物在冷却后会转成铁磁性:244CmN和244CmAs于109 K,248CmP于73 K,248CmSb于162 K。锔的镧系同类物钆以及钆的氮族元素化合物也会在冷却时转变磁性,但稍有不同:Gd和GdN变为铁磁性,而GdP、GdAs和GdSb则具反铁磁性。[26]
锔的电阻率会随温度而变化:在4至60 K时大约翻倍,并从60 K到室温几乎保持恒等。由于其释放的α辐射会破坏自身的晶体结构,因此其电阻率会随时间快速提高,约10 µOhm·cm/h。故此很难确定锔的绝对电阻率(约125 µΩ·cm)。锔的电阻率与钆、钚和镎相近,但比镅、铀、钋和钍高出许多。[6]
在紫外线照射下,锔(III)离子会发出强烈且稳定的橘黄色萤光,极值位于590至640 nm区间内,随环境条件而变化。[27]这种萤光特性是来自第一激发态6D7/2与基态8S7/2之间的转变。通过分析发出的萤光,可以监测有机及无机配合物中Cm(III)离子间的交互作用。[28]
锔的最稳定氧化态为+3,其离子在溶液中也具有+3态。[29]:24其+4态只出现在少有的几个固态化合物中,如CmO2和CmF4。[30][31]锔的化学特性与同为锕系元素的钍和铀不同,但和镅及许多镧系元素相似。在水溶液中,Cm3+离子可以是无色或浅绿色的,[32]而Cm4+离子则是浅黄色的。[33]Cm3+的吸收光谱在375.4、381.2和396.5纳米波长处有尖锐的峰值,这些峰值的强度可以直接用来测量该离子的浓度。[29]:25-26锔离子属于硬酸,因此可以和硬碱产生最稳定的配合物。[34]两者间形成的主要为离子键,但含少量共价键的部分。[35]配合物中的锔主要以三帽三角菱柱形配位。[36]
化合物及反应
锔会和氧迅速反应,主要形成Cm2O3和CmO2,[37]另也会形成二价氧化锔:CmO。[33]:1972草酸锔(Cm2(C2O4)3)、硝酸锔(Cm(NO3)3)或氢氧化锔在纯氧中燃烧后可制成呈黑色的CmO2。[31][38]当在真空中(约0.01 Pa压力下)加热到约600至650 °C度时,该氧化物会转变成呈白色的Cm2O3:[31][39]
另一种制得Cm2O3的方法是使用氢气对CmO2进行还原反应:[40]
其他已知的氧化物还包括诸如M(II)CmO3型的三元氧化物,其中的M表示任何一种二价金属,如钡。[41]
Remove ads
对含有三价锔离子的溶液注入氟离子,可产生无色的三氟化锔(CmF3)。呈棕色的四氟化锔(CmF4)则只能通过三氟化锔和氟气间的反应才能形成:[9]
锔还可以形成A7Cm6F31型的三元氟化物,其中的A表示碱金属。[42]
氢氧化锔(III)(Cm(OH)3)与无水氯化氢气体反应后,会形成无色的三氯化锔(CmCl3)。要制造其他的锔卤化物,可在约400至450 °C的高温下,使三氯化锔与对应的卤化铵盐反应。以此方法可制得三溴化锔(无色至浅绿色)及三碘化锔(无色):[43]
另一种方法须把氧化锔和对应的酸一起加热到600 °C(比如,要制造溴化锔,则要使用氢溴酸)。[44][45]对三氯化锔进行气态水解后,会产生氯氧化锔:[46]
Remove ads
在高温下真空中使锔与气态硫、硒或碲反应,可分别制成锔的硫化物、硒化物和碲化物。[47][48]氮族的氮、磷、砷和锑可以和锔形成化学式为CmX的化合物。[9]要制造这些化合物,可在高温下使三氢化锔(CmH3)或金属锔与对应的氮族元素进行反应。

钍、镤、镎、钚和镅等锕系元素都具有类似于双(环辛四烯)合铀的金属有机配合物。分子轨道理论预测存在双(环辛四烯)合锔 (η8-C8H8)2Cm,但至今仍待实验证明。[49][50]
延伸X光吸收细微结构(EXAFS)已证实,在含有n-C3H7-BTP和Cm3+离子的溶液中,存在Cm(n-C3H7-BTP)3型的配合物,其中BTP指2,6-二(1,2,4-三嗪-3-基)吡啶。某些BTP型配合物只和锔相互作用,因此在提取锔的过程中相当有用。[27][51]溶解了的Cm3+离子会和许多有机化合物反应,包括异羟肟酸、[52]尿素、[53]萤光素、[54]和三磷酸腺苷等。[55]这些化合物都和各种微生物的内部活动相关。如此产生的配合物在紫外线的照射激发下,会发出强烈的橘黄色萤光。这不但使锔的探测过程更为方便,更可以通过观测萤光寿命的改变(约0.1毫秒数量级)及光谱的变化,来研究Cm3+离子与配体间的交互作用。[28][52][53][54][55]
锔在生物体中没有已知的用途。[56]一些报告曾表明,细菌和古菌会吸附Cm3+离子,但锔并没有掺入这些生物体内。[57][58]
同位素
| 热中子截面(靶恩)[59] | ||||||
|---|---|---|---|---|---|---|
| 242Cm | 243Cm | 244Cm | 245Cm | 246Cm | 247Cm | |
| 裂变 | 5 | 617 | 1.04 | 2145 | 0.14 | 81.90 |
| 捕获 | 16 | 130 | 15.20 | 369 | 1.22 | 57 |
| 捕获/裂变比 | 3.20 | 0.21 | 14.62 | 0.17 | 8.71 | 0.70 |
| 53 MWd/kg的燃烧20年后的低浓缩铀乏核燃料[60] | ||||||
| 3种常见同位素 | 51 | 3700 | 390 | |||
| 快中子反应堆中的混合氧化物核燃料(5个样本的平均值,燃烧度为66-120GWd/t)[61] | ||||||
| 总含锔量3.09×10-3% | 27.64% | 70.16% | 2.166% | 0.0376% | 0.000928% | |
| 同位素 | 242Cm | 243Cm | 244Cm | 245Cm | 246Cm | 247Cm | 248Cm | 250Cm |
| 临界质量(kg) | 25 | 7.5 | 33 | 6.8 | 39 | 7 | 40.4 | 23.5 |
锔约有20种已知的同位素及7种同核异构体,质量数从233到252不等。这些同位素都具有放射性,其中半衰期最长的有247Cm(1560万年)和248Cm(348,000年);其他长半衰期的同位素包括245Cm(8500年)、250Cm(8,300年)和246Cm(4,760年)。锔-250较为特殊,主要以自发裂变的形式衰变(86%几率)。最常用的锔同位素为242Cm和244Cm,半衰期分别为162.8天和18.11年。[14]
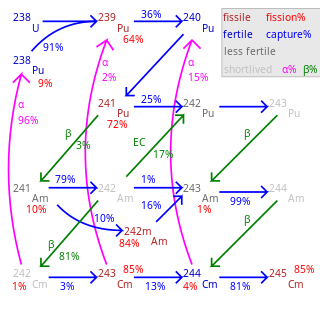
所有242Cm和248Cm之间的同位素,以及250Cm,都可以维持核链反应,因此理论上能在反应堆中作核燃料之用。正如多数超铀元素一样,质量数为奇数的锔同位素243Cm、245Cm和247Cm的核裂变截面特别高,都可以被用在热中子反应堆中。然而,所有锔同位素的混合物则只能用于快中子增殖反应堆中,因为质量数为偶数的锔同位素在热中子反应堆中不会裂变,并会随燃烧度的提高而累积。[63]当在发电反应堆中使用混合氧化物(MOX)燃料时,该燃料不应含有锔元素。这是因为中子活化会使248Cm变为锎,而锎是一种强中子射源,除了会污染核燃料循环的后部,还会对反应堆操作人员造成过量辐射的危险。因此,若要使用次锕系元素(铀和钚以外的锕系元素)作为热中子反应堆中的核燃料,则应从燃料中完全移除锔元素,或将锔置于特殊的燃料柱中作为燃料中的唯一一种锕系元素。[64]
右表列出锔同位素做成球体,且不使用减速剂或反射器时的临界质量。如加上金属反射器(厚30 cm的钢铁),则奇数同位素的临界质量大约为3至4 kg。如使用水(厚度约20至30 cm)作为反射器,则临界质量可以大大下降:245Cm可降至59克,243Cm为155克,而247Cm为1550克。这些数值具有很高的不确定度。[63][65]
由于产量稀少,造价昂贵,目前锔并没有被用作核燃料。[66]245Cm和247Cm的临界质量很小,理论上可用于制造便携核武器,但至今没有相关的报导。锔-243的半衰期很短,会产生过量热量,因此不可用于核武器中。[67]锔-247的半衰期是钚-239的647倍,因此很适合用来制造核武器。
Remove ads
存量

锔-247是锔同位素中半衰期最长的,有1560万年,但仍远短于地球的年龄。因此,所有原始的锔元素,也就是在地球形成时可能存在的锔,至今都已全部衰变殆尽。陨石Curious Marie中含有过量的235U,其为247Cm衰变产生的,是它曾存在于早期太阳系的痕迹。[68]铀矿中的铀原子经中子捕获及β衰变,有可能产生痕量的242Cm(238U → 239Pu → 240Pu → 241Am → 242Cm),但该反应未被证实。计算结果认为该反应所产生的242Cm的量极少,即使往大了算,1×108公斤、含18%铀的沥青铀矿里含有的242Cm仍少于一粒原子。[69][70][71]宇宙射线有可能把痕量的247Cm带到地球,但这同样未被证实。[69]自然界含有的244Pu有可能经双β衰变变成244Cm,但这一过程尚未被发现。[69][72]
现今地球上的锔大多出现在乏核燃料中,其余则是通过人工制造的,主要用于科学研究。1945至1980年大气层核试验的进行地点存有一定的锔元素。[37]美国第一颗氢弹“常春藤麦克”(1952年11月1日于埃内韦塔克环礁引爆)的辐射落尘中,除了含有锿、镄、钚和镅之外,还有锫、锎和锔的一些同位素,其中包括245Cm、246Cm及更少量的247Cm、248Cm和249Cm。由于正值冷战时期,这些结果起初被列为军事机密,到1956年才正式公布。[73]
大气层中的锔化合物较难溶于常见的溶剂中,但会黏附在泥土粒子上。分析表明,沙粒表面上的含锔量比其周围的水高出大约4,000倍;壤土中该比例甚至可高达18,000倍。[74]
从95号镅至100号镄的超铀元素,包括锔,都曾在位于加蓬奥克洛的天然核反应堆中自然产生,但至今已不再形成了。[75]普瑞兹毕尔斯基星的光谱可能存在锔与其它锕系元素。[76]
Remove ads
合成
锔是在核反应堆中少量产生的。到目前为止,242Cm和244Cm的总产量只有几公斤,其余更重的同位素只有数克或甚至数毫克的总产量。故此锔的价格昂贵,每毫克160至185美元;[16]更近期的估价为:242Cm每克2,000美元,244Cm每克170美元。[77]在反应堆中,238U可以通过一系列的核反应形成锔。首先238U捕获一颗中子,变为239U,再经β–衰变形成239Np和239Pu。
- (箭头下的时间为半衰期)
进一步捕获中子并进行β–衰变后,样本会变为241Am,再转换为242Cm:
实际研究在制造锔的时候,并不对铀进行照射,而是用钚。乏核燃料中含有大量的钚,能够轻易地提取使用。如果使用高中子通量的辐射,能通过另一条反应链形成244Cm:[9]
锔-244在释放α粒子后,会衰变成240Pu;同时它会吸收中子,产生少量更重的锔同位素。这些同位素包括247Cm和248Cm,由于半衰期很长,因此常被用于科学研究。不过,由于247Cm在热中子撞击下容易裂变,因此该同位素在热中子反应堆中的产率较低。[78]经中子捕获产生250Cm的可能性同样很低,因为中间产物249Cm的半衰期非常短(64分钟),并会经β–衰变成为锫的249Bk同位素。[78]
- (A = 244–248)
以上一连串的(n,γ)反应会产生不同锔同位素的混合物。合成后的分离过程十分繁复,所以科学家一般选择性地合成特定锔同位素。由于半衰期很长,锔-248最常用于研究用途。该同位素最有效率的合成方法是通过锎-252的α衰变。因为252Cf具有长半衰期(2.65年),因此容易大量取得。每年通过这种方法产生的248Cm大约有35至50 mg,同位素纯度为97%。[78]
另一种研究常用的同位素245Cm可经由249Cf的α衰变产生,而249Cf可由249Bk的β–衰变产生。

一般的合成产物含有不同锔同位素的氧化物混合物。要分离出其中一种同位素,可以将乏核燃料(如混合氧化物核燃料)溶于硝酸中,再使用磷酸三丁酯和烃类的混合物,通过铀钚分离(PUREX)来萃取出大部分的铀和钚。然后利用二酰胺来萃取水溶残余物(残液)中剩余的镧系元素和锕系元素。产物将会是三价锕系及镧系元素的混合物。要分离出当中的锔化合物,可用多重步骤的层析法及离心法,并使用适当的试剂。[29]:34-48其中一种可用来专门提取锔的试剂为双三嗪基二吡啶配合物。[79]如要使锔从非常相似的镅中分离出来,可将两者氢氧化物的混合浆状物置于碳酸氢钠水溶液中,并在加热后加入臭氧。大部分的镅和锔在溶液中都具有+3价态。其中镅会被氧化,形成可溶的Am(IV)配合物,而锔则不会改变,故可再重复用离心法提取出来。[29]:25
科学家通过对锔化合物进行还原反应来取得处于金属态的锔元素。其中一种可用于制备锔金属的化合物为三氟化锔。反应必须在不含水或氧的环境下进行,使用钽和钨造的器具,并以钡或锂作为还原剂。[9][19][80][81][82]
应用

锔是其中一种放射性最强的可分离元素。其两种最常见的同位素242Cm和244Cm都是强α粒子射源(能量为6 MeV),其半衰期相对较短,分别为162.8天和18.1年,每克所释放的功率分别为120瓦和3瓦。[16][84][85]因此氧化锔可被用于太空船中的放射性同位素热电机。科学家曾研究过如何用244Cm同位素发电,而242Cm则因价格昂贵(每克约2000美元)而不能使用。锔-243的半衰期约为30年,每克功率达到1.6瓦,故可用作燃料,但它的核衰变产物会释放大量有害的γ和β射线。244Cm所释放的α粒子无须大量辐射防护,但其自发裂变率很高,因此具有高中子辐射和γ辐射。相比同样用于放射性同位素热电机的238Pu,244Cm释放的中子通量高出500倍;它释放强烈的γ射线,所需的辐射防护也高出20倍。功率为1 kW的样本需要约5 cm的铅作防护,而238Pu只需0.1 cm的铅。这样的应用在目前来说是不切实际的。[77]
其中一项锔的实际应用是利用242Cm同位素来产生心律调节器中用于发电的238Pu。如不使用这种方法,则要通过237Np的(n,γ)反应,或用氘撞击铀,才能形成238Pu。这些过程都会产生236Pu,而这种副产品会衰变为释放大量γ辐射的208Tl,不适合加入心律调节器中。[86]
科学家常使用锔来产生更重的超铀元素和锕系后元素。用氧(18O)或镁(26Mg)撞击248Cm,可以产生𬭳(265Sg)和𬭶(269Hs和270Hs)。[33]:1980-1981劳伦斯伯克利国家实验室用能量为35 MeV的α粒子撞击重数微克的锔-242,发现了锎元素:
- 242
96Cm
+ 4
2He
→ 245
98Cf
+ 1
0
n

同位素244Cm最实际的用途是在α粒子X射线光谱仪(APXS)中作α粒子射源,但可用体积有限。火星探路者、火星车、火星96、勇气号、火星探测漫游者、机遇号和火星科学实验室都使用了这种仪器来分析火星表面岩石的成分和结构。[87]测量员5至7号月球探测器也使用了APXS,但所用的α粒子源是242Cm。[74][88][89]
APXS上装有一个传感器头,里面含有6个锔α粒子源,其总放射性衰变率为几十毫居里(约十亿贝可勒尔)。射源对准样本后,仪器就会分析从样本散射出来的α粒子和质子的能谱(只有某些光谱仪有分析质子的功能)。这些能谱包含有关所有主要元素量的信息(氢、氦和锂除外)。[90]罗塞塔号的菲莱登陆器(Philae Lander)也将用APXS来探测楚留莫夫-格拉希门克彗星的表面。[91]
安全
由于具有放射性,锔必须在适当的实验室中用特殊的器材处理。锔元素本身主要释放α粒子,用很薄的普通材质就可以吸收阻挡。然而锔的一些衰变产物却会释放大量β及γ辐射,因此需要更加严密的保护措施。[37]一旦摄入体内,锔会在几天以内被排除体外,只剩余0.05%会吸收到血液内。血液中45%的锔会进入肝脏,45%进入骨骼,余下10%经排泄离开身体。骨骼中的锔会积累在与骨组织与骨髓的接触面上,而且不会随时间明显地分散。其辐射会破坏骨髓和其制造红血球的能力。锔的生物半衰期在肝脏中约为20年,而在骨骼中则为50年。[37][74]锔更容易通过呼吸进入体内,其中244Cm在水溶态时的最高允许可摄入量为0.3 μC。[16]含242Cm和244Cm的溶液在经静脉注射进入老鼠的体内后,会导致其患上骨肿瘤的可能增大;经吸入后,则有可能造成肺癌和肝癌。[37]
乏核燃料中不可避免地会含有锔同位素,大约每吨含20克锔。[92]其中245Cm至248Cm同位素的半衰期有数千年之久,必须先从要弃置的乏核燃料中分离出来。[93]锔分离出来后,要在反应堆中经中子撞击,成为短半衰期的核素。这种方法称为核嬗变,科学家目前正在研发锔的核转化过程。[27]
参考资料
书目
外部链接
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads






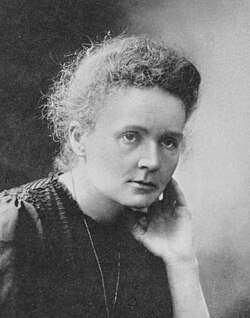






![{\displaystyle \mathrm {^{238}_{\ 92}U\ \xrightarrow {(n,\gamma )} \ _{\ 92}^{239}U\ {\xrightarrow[{23.5\ min}]{\beta ^{-}}}\ _{\ 93}^{239}Np\ {\xrightarrow[{2.3565\ d}]{\beta ^{-}}}\ _{\ 94}^{239}Pu} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/7547ff8c33f18d47ae71f22e764531f67037b5f5)
![{\displaystyle \mathrm {^{239}_{\ 94}Pu\ \xrightarrow {2(n,\gamma )} \ _{\ 94}^{241}Pu\ {\xrightarrow[{14.35\ yr}]{\beta ^{-}}}\ _{\ 95}^{241}Am\ \xrightarrow {(n,\gamma )} \ _{\ 95}^{242}Am\ {\xrightarrow[{16.02\ h}]{\beta ^{-}}}\ _{\ 96}^{242}Cm} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/82ed2cb97755d2422bf395cace336734f3517b61)
![{\displaystyle \mathrm {^{239}_{\ 94}Pu\ \xrightarrow {4(n,\gamma )} \ _{\ 94}^{243}Pu\ {\xrightarrow[{4,956\ h}]{\beta ^{-}}}\ _{\ 95}^{243}Am\ \xrightarrow {(n,\gamma )} \ _{\ 95}^{244}Am\ {\xrightarrow[{10.1\ h}]{\beta ^{-}}}\ _{\ 96}^{244}Cm} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/d4fbdb09a82cafad566e2e91ba722701d1c658b4)
![{\displaystyle \mathrm {^{244}_{\ 96}Cm\ {\xrightarrow[{18.11\ yr}]{\alpha }}\ _{\ 94}^{240}Pu} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/5ae7eb97e7c5f25d3e31d060e2a634d5a8f547f6)

![{\displaystyle \mathrm {^{252}_{\ 98}Cf\ {\xrightarrow[{2.645\ yr}]{\alpha }}\ _{\ 96}^{248}Cm} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/c414a0a8639a7d00067903f990993273d718ce8c)
![{\displaystyle \mathrm {^{249}_{\ 97}Bk\ {\xrightarrow[{330\ d}]{\beta ^{-}}}\ _{\ 98}^{249}Cf\ {\xrightarrow[{351\ yr}]{\alpha }}\ _{\ 96}^{245}Cm} }](http://wikimedia.org/api/rest_v1/media/math/render/svg/1423d2e5aa217ad71886152bf7fcd6c204eccd43)
