DOx
Class of chemical compounds From Wikipedia, the free encyclopedia
4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring.[1][2] They are 4-substituted derivatives of 2,5-dimethoxyamphetamine (2,5-DMA, DOH) and are structurally related to the naturally occurring phenethylamine psychedelic mescaline.

The most well-known DOx drugs are DOM, DOI, DOB, DOET, and DOC.[3] DOI is widely used in scientific research.[2][4] DOM has been used as a recreational drug, while DOET was an experimental pharmaceutical drug.[5]
Most compounds of this class are potent and long-lasting psychedelic drugs, and act as selective 5-HT2A, 5-HT2B, and 5-HT2C receptor agonists.[6][7] A few bulkier derivatives such as DOAM have similarly high affinity for 5-HT2 receptors but have reduced activational efficacy and do not produce psychedelic effects.[2][6]
DOI has been found to have extraordinarily potent anti-inflammatory effects.[8][9][10] These properties are not shared by all other related drugs and appear to be mediated by functionally selective serotonin 5-HT2A receptor activation.[9][11] The anti-inflammatory effects of DOI and related drugs may have medical applications.[8][9]
Use
| Compound | Chemical name | Dosage | Duration | |
|---|---|---|---|---|
| DOAM | 4-Amyl-2,5-dimethoxyamphetamine | ≥5–40 mg | Unknown | |
| DOB | 4-Bromo-2,5-dimethoxyamphetamine | 1–3 mg | 18–30 hours | |
| DOBU | 4-Butyl-2,5-dimethoxyamphetamine | ≥1–10 mg | Very long | |
| DOBz (DOBN) | 4-Benzyl-2,5-dimethoxyamphetamine | Unknown | Unknown | |
| DOC | 4-Chloro-2,5-dimethoxyamphetamine | 1.5–5 mg | 12–24 hours | |
| DOEF | 4-(2-Fluoroethyl)-2,5-dimethoxyamphetamine | 2–3.5 mg | 12–16 hours | |
| DOET | 4-Ethyl-2,5-dimethoxyamphetamine | 2–6 mg | 5–20 hours | |
| DOF | 4-Fluoro-2,5-dimethoxyamphetamine | >18 mg | Unknown | |
| DOH (2,5-DMA) | 2,5-Dimethoxyamphetamine | 80–160 mg | 6–8 hours | |
| DOHx | 4-Hexyl-2,5-dimethoxyamphetamine | Unknown | Unknown | |
| DOI | 4-Iodo-2,5-dimethoxyamphetamine | 1.5–3 mg | 16–30 hours | |
| DOIB | 4-Isobutyl-2,5-dimethoxyamphetamine | 10–15 mg | Unknown | |
| DOIP | 4-Isopropyl-2,5-dimethoxyamphetamine | 20–30 mg | Unknown | |
| DOM | 4-Methyl-2,5-dimethoxyamphetamine | 3–10 mg | 14–20 hours | |
| DON | 4-Nitro-2,5-dimethoxyamphetamine | 3–4.5 mg | 8–15 hours | |
| DOPP | 4-(3-Phenylpropyl)-2,5-dimethoxyamphetamine | Unknown | Unknown | |
| DOPR | 4-Propyl-2,5-dimethoxyamphetamine | 2.5–5 mg | 20–30 hours | |
| DOSB | 4-sec-Butyl-2,5-dimethoxyamphetamine | 25–30 mg | Very long | |
| DOTB | 4-tert-Butyl-2,5-dimethoxyamphetamine | >25 mg | Unknown | |
| DOTFE | 4-(2,2,2-Trifluoroethyl)-2,5-dimethoxyamphetamine | >3 mg | Unknown | |
| DOTFM | 4-(Trifluoromethyl)-2,5-dimethoxyamphetamine | 0.3–1 mg | Unknown | |
| DOYN | 4-Ethynyl-2,5-dimethoxyamphetamine | 2–6 mg | 10–15 hours | |
| MEM | 4-Ethoxy-2,5-dimethoxyamphetamine | 20–50 mg | 10–14 hours | |
| Aleph (DOT) | 4-Methylthio-2,5-dimethoxyamphetamine | 5–10 mg | 6–8 hours | |
| Aleph-2 | 4-Ethylthio-2,5-dimethoxyamphetamine | 4–8 mg | 8–16 hours | |
| Aleph-4 | 4-Isopropylthio-2,5-dimethoxyamphetamine | 7–12 mg | 12–20 hours | |
| Aleph-6 | 4-Phenylthio-2,5-dimethoxyamphetamine | ≥40 mg | Very long | |
| Aleph-7 | 4-Propylthio-2,5-dimethoxyamphetamine | 4–7 mg | 15–30 hours | |
| G-1 | 3,4-Dimethyl-2,5-dimethoxyamphetamine | 20–32 mg | 18–24 hours | |
| G-3 | 3,4-(Trimethylene)-2,5-dimethoxyamphetamine | 12–18 mg | 8–12 hours | |
| G-5 | 3,4-Norbornyl-2,5-dimethoxyamphetamine | 14–20 mg | 16–30 hours | |
| TMA-2 (2,4,5-TMA) | 4-Methoxy-2,5-dimethoxyamphetamine | 20–40 mg | 8–12 hours | |
| Refs: [2][12][13][3][14][15][16][17][18][19][20][21][22][23] | ||||
Side effects
DOx drugs like DOM have been associated with certain side effects that have not occurred to the same extent with other psychedelics like LSD.[5] Examples of such side effects include physical symptoms like sweating, tremors, and large increases in heart rate.[5]
Interactions
Pharmacology
Summarize
Perspective
Pharmacodynamics
Actions
| Compound | Affinity (Ki, nM) | ||
|---|---|---|---|
| 5-HT2A | 5-HT2B | 5-HT2C | |
| 2,5-DMA | 211–2,502 | 1,039 | 104–>5,070 |
| DOM | 88–507.4 | 11.7 | 404–3,980 |
| DOET | 12–100 | 28.8 | 107.2–108 |
| DOPR | 0.9 | 54.4 | 1.1 |
| DOBU | 5.4 | ND | 60 |
| DOTB | 3.7 | 24.6 | 2.2 |
| DOAM | 3.5 | ND | 75 |
| DOHx | 0.1 | 30.3 | 0.7 |
| DOF | 41.7 | 227 | 28.7 |
| DOC | 1.4 | 31.8 | 2.0 |
| DOB | 0.6–41 | 26.9 | 1.3–60 |
| DOI | 0.7–165.4 | 20.0–335.9 | 2.4–45.8 |
| TMA-2 | 57.9–584.2 | 154.4–307 | 87.7–4,062 |
| MEM | 73.0–3,948 | 64.5–763 | 124–>10,000 |
| Aleph-2 | 60.4 | 1.6 | 50.3 |
| DOAc | 80.5 | 313 | 91.3 |
| DON | 5.5 | 166 | 22.4 |
| DOCN | 45.7 | 774 | 1,011 |
| DOBZ | 0.4 | 35.0 | 1.0 |
| M-154 | 94.2 | 341 | 68.1 |
| D-367 | 88.5 | 521 | 514 |
| QDOB | 2,155 | >10,000 | 6,298 |
| Notes: The smaller the value, the more avidly the drug binds to the site. Refs: [24][25][7][6][26][27] | |||
The DOx drugs act as agonists of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.[6][28][7][2][3][29] Their psychedelic effects are thought to be mediated specifically by activation of the serotonin 5-HT2A receptor.[28][2]
In contrast to other amphetamines, DOx drugs like DOC, DOET, and DOM are inactive as monoamine releasing agents and reuptake inhibitors.[30][31][6] Some of the DOx drugs, including DOB, DOET, DOI, and DOM, are agonists of the rat, rhesus monkey, and/or human trace amine-associated receptor 1 (TAAR1) with varying potencies.[32][33]
Effects
In contrast to amphetamines like (–)-cathinone, but similarly to mescaline, DOM has shown no stimulant-like or reinforcing effects in rhesus monkeys.[34][35][36][37] Conversely however, DOC has shown reinforcing effects, including conditioned place preference (CPP) and self-administration, in rodents similarly to methamphetamine.[38] This is analogous to other findings in which various 2C and NBOMe drugs have been found to produce brain dopaminergic elevations and reinforcing effects in rodents.[39][40][41][42][43][44][45]
Pharmacokinetics
The DOx drugs are orally active and many have doses in the range of 1 to 10 mg and durations in the range of 8 to 30 hours.[14][3][2][12][5] Some DOx drugs, such as DOM and DOB, appear to have durations that increase non-linearly with dosage, for instance 8 hours at lower doses and as long as 30 hours or even up to 3 or 4 days at higher doses.[5][15] This suggests that the pathways mediating the metabolism of these drugs can saturate.[5] The DOx drugs are metabolized primarily by O-demethylation.[3] However, DOM is primarily metabolized by hydroxylation at its methyl group.[3]
History
Summarize
Perspective
DOM was the first psychedelic of the DOx series to be discovered.[4] It was first synthesized by Alexander Shulgin at Dow Chemical Company in 1963, who had had his first psychedelic experience, with mescaline (3,4,5-trimethoxyphenethylamine), in 1960.[4][5][16] Shulgin personally tried DOM on January 4, 1964 and discovered its psychedelic effects.[46][4][5][16] 2,4,5-Trimethoxyamphetamine (TMA-2; "DOMeO") had been synthesized by Bruckner in 1933, but its psychedelic effects were not described until Shulgin tried the compound and reported its effects in the scientific literature in 1964.[22][47][48] Prior to this, 3,4,5-trimethoxyamphetamine (TMA; α-methylmescaline) had been synthesized by Hey in 1947, being found by him to produce euphoria, and was described by Peretz and colleagues in 1955 as clearly producing psychedelic effects.[22][49][50][51]
Following his discovery of DOM, Shulgin developed DOET and found that at low doses it was a remarkable "psychic energizer" without producing psychedelic effects at these doses.[5] Dow Chemical Company decided to move forward with clinical trials of DOET as a potential pharmaceutical drug for such purposes.[5] Shulgin and Dow Chemical Company filed a patent for DOET in 1966, although it was not published until 1970.[5][4][52] Dow Chemical Company tasked Solomon H. Snyder at Johns Hopkins University with clinically studying DOET.[5]
In April 1967, following the banning of LSD in California in 1966, DOM emerged as a street drug and legal LSD alternative with the name "STP" (allegedly short for "Serenity, Tranquility, and Peace") in the Haight-Ashbury district in San Francisco.[5][53] This occurred due to DOM being publicly distributed for free in the form of high-dose tablets by LSD distributor Owsley Stanley, who had personally learned of DOM from Shulgin.[5][53] It is unclear why Shulgin provided information about DOM to Stanley, since doing so had the potential to risk Shulgin's professional career and the DOET clinical studies.[5][53] One possibility is that Dow Chemical Company was not further looking into DOM and Shulgin thought that it was a promising drug that would otherwise be forgotten.[5] In any case, street use of DOM was short-lived because the tablets caused a public health crisis due to them often producing very long durations (up to 3–4 days), intense experiences, worrying physical side effects, and hospitalizations.[5] DOM was first reported on in the media and scientific literature in 1967 as a result of the crisis.[5][54][55] DOM became illegal in the United States in 1968.[5]
Dow Chemical Company terminated its clinical research program on DOET due to the DOM public health crisis.[5] DOET was subsequently first described in the literature by Snyder and colleagues in 1968.[55] Snyder continued to be interested in DOET as a potential medicine, but it was never further developed.[55] Snyder also described 2,5-dimethoxyamphetamine (2,5-DMA), which had been synthesized and tested by Shulgin, in the literature in 1968.[56] DOM and DOET were further described in the scientific literature by Shulgin in 1969.[57][4][5] In addition, Shulgin discussed DOM, DOET, TMA-2, and 2,5-DMA in a book chapter on hallucinogens published in 1970.[58]
The earlier DOx drugs like DOM and DOET were subsequently followed by DOB, which was developed by Shulgin and colleagues like Claudio Naranjo, in 1971,[4][59] and by DOI, DOC, and a few other analogues, which were developed by another research group, in 1973.[4][60] After this, numerous other DOx drugs were synthesized and characterized, both by Shulgin and other scientists.[22][13][15][12][16][61][2]
Following its discovery, DOI has become widely used in scientific research in the study of the serotonin 5-HT2 receptors.[4][2]
List of DOx drugs
Summarize
Perspective
The DOx family includes the following members:
| Structure | Name | Abbreviation | CAS # | Ref |
|---|---|---|---|---|
 |
2,5-Dimethoxyamphetamine | 2,5-DMA | 2801-68-5 | |
 |
2,5-Dimethoxy-4-amylamphetamine | DOAM | 63779-90-8 | |
 |
2,5-Dimethoxy-4-bromoamphetamine | DOB | 64638-07-9 | |
 |
2,5-Dimethoxy-4-butylamphetamine | DOBU | 63779-89-5 | |
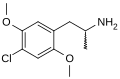 |
2,5-Dimethoxy-4-chloroamphetamine | DOC | 123431-31-2 | |
 |
2,5-Dimethoxy-4-ethoxyamphetamine | MEM | 16128-88-4 | |
 |
2,5-Dimethoxy-4-(methoxymethyl)amphetamine | DOMOM | 260810-10-4 | [62] |
 |
2,5-Dimethoxy-4-(ethoxymethyl)amphetamine | DOMOE | 930836-81-0 | |
 |
2,5-Dimethoxy-4-ethylamphetamine | DOET | 22004-32-6 | |
 |
2,5-Dimethoxy-4-ethylthioamphetamine | Aleph-2 | 185562-00-9 | |
 |
2,5-Dimethoxy-4-fluoroamphetamine | DOF | 125903-69-7 | |
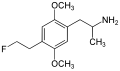 |
2,5-Dimethoxy-4-(2-fluoroethyl)amphetamine | DOEF | 121649-01-2 | |
 |
2,5-Dimethoxy-4-(3-fluoropropyl)amphetamine | DOPF | ? | |
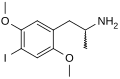 |
2,5-Dimethoxy-4-iodoamphetamine | DOI | 42203-78-1 | |
 |
2,5-Dimethoxy-4-isopropylthioamphetamine | Aleph-4 | 123643-26-5 | |
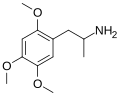 |
2,4,5-Trimethoxyamphetamine | TMA-2 (DOMeO) | 1083-09-6 | |
 |
2,5-Dimethoxy-4-methylamphetamine | DOM | 15588-95-1 | |
 |
2,5-Dimethoxy-4-methylthioamphetamine | Aleph-1 (DOT) | 61638-07-1 | |
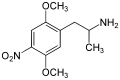 |
2,5-Dimethoxy-4-nitroamphetamine | DON | 67460-68-8 | |
 |
2,5-Dimethoxy-4-phenylthioamphetamine | Aleph-6 | 952006-44-9 | |
 |
2,5-Dimethoxy-4-benzylamphetamine | DOBz | 125903-73-3 | |
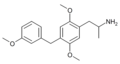 |
2,5-Dimethoxy-4-(3-methoxybenzyl)amphetamine | DO3MeOBZ | 930836-90-1 | [63] |
 |
2,5-Dimethoxy-4-[(tetrahydrofuran-2-yl)methyl]amphetamine | DOTHFM | 930776-12-8 | |
 |
2,5-Dimethoxy-4-propylamphetamine | DOPR | 63779-88-4 | |
 |
2,5-Dimethoxy-4-isopropylamphetamine | DOiP | 42306-96-7 | |
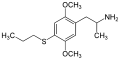 |
2,5-Dimethoxy-4-propylthioamphetamine | Aleph-7 | 207740-16-7 | |
 |
2,5-Dimethoxy-4-(difluoromethyl)amphetamine | DODFM | ? | |
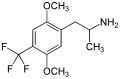 |
2,5-Dimethoxy-4-trifluoromethylamphetamine | DOTFM | 159277-07-3 | |
 |
2,5-Dimethoxy-4-(2,2,2-trifluoroethyl)amphetamine | DOTFE | ? | [64] |
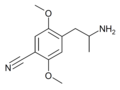 |
2,5-Dimethoxy-4-cyanoamphetamine | DOCN | 125903-74-4 | [65] |
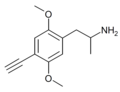 |
2,5-Dimethoxy-4-ethynylamphetamine | DOYN | 633290-70-7 | [66] |
 |
2,5-Dimethoxy-4-acetylamphetamine | DOAc | ? | |
 |
2,5-Dimethoxy-4-isobutylamphetamine | DOIB | 89556-64-9 | |
 |
2,5-Dimethoxy-4-sec-butylamphetamine | DOSB | 89556-71-8 | |
 |
2,5-Dimethoxy-4-tert-butylamphetamine | DOTB | 41538-42-5 | |
 |
2,5-Dimethoxy-4-hexylamphetamine | DOHx | ? | |
 |
2,5-Dimethoxy-4-octylamphetamine | DOCT | ? | |
 |
4-(3-Phenylpropyl)-2,5-dimethoxyamphetamine | DOPP | ? | |
| 4-Methallyloxy-2,5-dimethoxyamphetamine | MMALM | ? | [67] | |
| 4-Allyloxy-2,5-dimethoxyamphetamine | MALM | ? | [67] | |
| 4-(2-Fluoroethoxy)-2,5-dimethoxyamphetamine | MFEM | ? | [67] | |
| 4-(2,2-Difluoroethoxy)-2,5-dimethoxyamphetamine | MDFEM | ? | [67] | |
| 4-(2,2,2-Trifluoroethoxy)-2,5-dimethoxyamphetamine | MTFEM | ? | [67] | |
| 4-Isopropoxy-2,5-dimethoxyamphetamine | MIPM | ? | [67] | |
Related compounds
A number of additional compounds are known with alternative substitutions:
| Structure | Name | Abbreviation | CAS # |
|---|---|---|---|
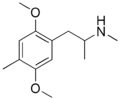 |
Dimethoxymethamphetamine ("Beatrice") | N-Methyl-DOM | 92206-37-6 |
 |
2,5-Dimethoxy-3,4-methylenedioxyamphetamine | DMMDA | 15183-13-8 |
 |
2,5-Dimethoxy-3,4-dimethylamphetamine ("Ganesha") | 3-Methyl-DOM | 207740-37-2 |
 |
2,5-Dimethoxy-3,4-trimethylenylamphetamine | G-3 | ? |
 |
2,5-Dimethoxy-3,4-tetramethylenylamphetamine | G-4 | ? |
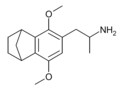 |
2,5-Dimethoxy-3,4-norbornylamphetamine | G-5 | ? |
 |
1,4-Dimethoxynaphthyl-2-isopropylamine | G-N | ? |
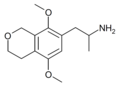 |
1-(5,8-Dimethoxy-3,4-dihydro-1H-isochromen-7-yl)propan-2-amine[68] | G-O | 774538-38-4 |
 |
2,5-Dimethoxy-3,4-dichloroamphetamine | DODC | 1373918-65-0 |
 |
2,5-Dimethoxy-4-iodo-N,N-dimethylamphetamine | IDNNA | 67707-78-2 |
 |
Methyl-DOB | N-Methyl-DOB | 155638-80-5 |
 |
2,3,4,5-Tetramethoxyamphetamine | TeMA | 23693-26-7 |
 |
1-(4-Bromo-2,3,6,7-tetrahydrofuro[2,3-f][1]benzofuran-8-yl)propan-2-amine | DOB-FLY | 219986-75-1 |
 |
Bromo-DragonFLY | DOB-DFLY | 502759-67-3 |
 |
2,5-Dimethoxy-4-methylphenylcyclopropylamine | DMCPA | 15183-13-8 |
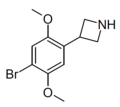 |
3-(4-Bromo-2,5-dimethoxyphenyl)azetidine | ZC-B[69] | 2641630-65-9 |
See also
- 2,5-Dimethoxyamphetamine
- Substituted mescaline analogue
- 2Cs, 4Cs, 25-NB, FLY
- Substituted amphetamines
- Substituted benzofurans
- Substituted cathinones
- Substituted methoxyphenethylamine
- Substituted methylenedioxyphenethylamines
- Substituted phenethylamines
- Substituted tryptamines
- PiHKAL
- The Shulgin Index
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
