Loading AI tools
Family of serotonergic psychedelics From Wikipedia, the free encyclopedia
The 25-NB (25x-NBx) series, sometimes alternatively referred to as the NBOMe compounds, is a family of serotonergic psychedelics.[1] They are substituted phenethylamines and were derived from the 2C family.[1] They act as selective agonists of the serotonin 5-HT2A receptor.[2][3][4][5][6][7][8] The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and relatively selective for the 5-HT2A receptor.[1] Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.[9]

NBOMe compounds are often associated with life-threatening toxicity and death.[10][11] Studies on NBOMe family of compounds demonstrated that the substance exhibit neurotoxic and cardiotoxic activity.[12] Reports of autonomic dysfunction remains prevalent with NBOMe compounds, with most individuals experiencing sympathomimetic toxicity such as vasoconstriction, hypertension and tachycardia in addition to hallucinations.[13][14][15][16][17] Other symptoms of toxidrome include agitation or aggression, seizure, hyperthermia, diaphoresis, hypertonia, rhabdomyolysis, and death.[13][17][11] Researchers report that NBOMe intoxication frequently display signs of serotonin syndrome.[18] The likelihood of seizure is higher in NBOMes compared to other psychedelics.[12]
NBOMe and NBOHs are regularly sold as LSD in blotter papers,[11][19] which have a bitter taste and different safety profiles.[13][10] Despite high potency, recreational doses of LSD have only produced low incidents of acute toxicity.[10] Fatalities involved in NBOMe intoxication suggest that a significant number of individuals ingested the substance which they believed was LSD,[15] and researchers report that "users familiar with LSD may have a false sense of security when ingesting NBOMe inadvertently".[13] While most fatalities are due to the physical effects of the drug, there have also been reports of death due to self-harm and suicide under the influence of the substance.[20][21][13]
Given limited documentation of NBOMe consumption, the long-term effects of the substance remain unknown.[13] NBOMe compounds are not active orally,[lower-alpha 1] and are usually taken sublingually.[1]: 3 When NBOMes are administered sublingually, numbness of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD.[23][24][25]
Many of the NBOMe compounds have high potency agonist activity at additional 5-HT receptors and prolonged activation of 5-HT2B can cause cardiac valvulopathy in high doses and chronic use.[11][16] 5-HT2B receptors have been strongly implicated in causing drug-induced valvular heart disease.[26][27][28] The high affinity of NBOMe compounds for adrenergic α1 receptor has been reported to contribute to the stimulant-type cardiovascular effects.[16]
In vitro studies, 25C-NBOMe has been shown to exhibit cytotoxicity on neuronal cell lines SH-SY5Y, PC12, and SN471, and the compound was more potent than methamphetamine at reducing the visibility of the respective cells; the neurotoxicity of the compound involves activation of MAPK/ERK cascade and inhibition of Akt/PKB signaling pathway.[12] 25C-NBOMe, including the other derivative 25D-NBOMe, reduced the visibility of cardiomyocytes H9c2 cells, and both substances downregulated expression level of p21 (CDC24/RAC)-activated kinase 1 (PAK1), an enzyme with documented cardiac protective effects.[12]
Preliminary studies on 25C-NBOMe have shown that the substance is toxic to development, heart health, and brain health in zebrafish, rats, and Artemia salina, a common organism for studying potential drug effects on humans, but more research is needed on the topic, the dosages, and if the toxicology results apply to humans. Researchers of the study also recommended further investigation of the drug's potential in damaging pregnant women and their fetus due to the substance's damaging effects to development.[29][30]
At present, there are no specific antidotes for NBOMes, and all acute intoxication is managed by symptomatic treatments, such as administration of benzodiazepines, antipsychotic drugs, and antiarrhythmic agents, such as beta blockers; some emergency interventions are intended to specifically treat rhabdomyolysis, which may lead to critical complications such as metabolic acidosis and acute kidney injury.[12]
The 25-NB compounds are mostly N-benzylphenethylamines,[1][31] though in some cases the phenyl ring of the N-benzyl group is replaced by other heterocycles such as thiophene, pyridine, furan, tetrahydrofuran, benzodioxole or naphthalene, among others.[32][33]
Generally speaking, they have methoxy groups at the 2 and 5 positions of the phenyl ring, a substitution such as a halogen or alkyl group at the 4 position of the phenyl ring, and a methoxy or other substitution (e.g., hydroxyl, fluoro) at the 2 position of the N-benzyl ring.[1] More rarely, other substitution patterns may be present [34][35] (see e.g. NBOMe-mescaline, 25G-NBOMe, 2CBFly-NBOMe, 25C-NB3OMe). They differ from the 2C series by the presence of the N-benzyl moiety.[1]
Rarely an alpha-methyl group is present making them N-benzyl amphetamines rather than N-benzyl phenethylamines, but this greatly reduces potency and activity. However in some cases where a side chain methyl group is cyclised back to the ring (e.g. in 2CBCB-NBOMe) or links the two alpha positions (e.g. in DMBMPP), this can improve selectivity for the 5-HT2A receptor subtype.[36]

This list includes notable compounds representative of most of the structural variations that have been explored in this series, but is by no means exhaustive. Many derivatives invented for scientific study into the structure-activity relationships of 5-HT2 receptor agonists have never appeared as designer drugs, while conversely some derivatives that have appeared as designer drugs are structurally novel and of unknown pharmacological activity (e.g. C30-NBOMe, 5-APB-NBOMe).
| Chemical structure | Common name | Chemical name | CAS number | R | R1 | Cyc |
|---|---|---|---|---|---|---|
 |
25B-NB | N-benzyl-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 155639-26-2 | 2,5-dimethoxy-4-bromo | H | phenyl |
 |
25C-NB | N-benzyl-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1391487-65-2 | 2,5-dimethoxy-4-chloro | H | phenyl |
 |
25I-NB | N-benzyl-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-18-5 | 2,5-dimethoxy-4-iodo | H | phenyl |
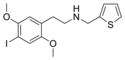 |
25I-NMeTh | N-[(thiophen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391499-03-8 | 2,5-dimethoxy-4-iodo | H | thiophen-2-yl |
 |
25B-NMePyr | N-[(pyridin-2-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391499-21-0 | 2,5-dimethoxy-4-bromo | H | pyridin-2-yl |
 |
25I-NMeFur | N-[(furan-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391498-93-3 | 2,5-dimethoxy-4-iodo | H | furan-2-yl |
 |
25I-NMeTHF | N-[(tetrahydrofuran-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | tetrahydrofuran-2-yl | |
 |
25B-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1539266-17-5 | 2,5-dimethoxy-4-bromo | H | 2-fluorophenyl |
 |
25B-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1335331-46-8 | 2,5-dimethoxy-4-bromo | H | 2-hydroxyphenyl |
 |
25B-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1026511-90-9 | 2,5-dimethoxy-4-bromo | H | 2-methoxyphenyl |
 |
25B-NB23DM | N-(2,3-dimethoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391493-68-7 | 2,5-dimethoxy-4-bromo | H | 2,3-dimethoxyphenyl |
 |
25B-NB25DM | N-(2,5-dimethoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 2,5-dimethoxy-4-bromo | H | 2,5-dimethoxyphenyl | |
 |
25B-NMe7BF | N-[(benzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-46-8 | 2,5-dimethoxy-4-bromo | H | benzofuran-7-yl |
 |
25B-NMe7DHBF | N-[(2,3-dihydrobenzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-40-2 | 2,5-dimethoxy-4-bromo | H | 2,3-dihydrobenzofuran-7-yl |
 |
25B-NMe7BT | N-[(benzothiophen-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-59-3 | 2,5-dimethoxy-4-bromo | H | benzothiophen-7-yl |
 |
25B-NMe7Box | N-[(benzoxazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-73-9 | 2,5-dimethoxy-4-bromo | H | benzoxazol-7-yl |
 |
25B-NMe7Ind | N-[(indol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-28-4 | 2,5-dimethoxy-4-bromo | H | indol-7-yl |
 |
25B-NMe7Indz | N-[(indazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-43-3 | 2,5-dimethoxy-4-bromo | H | indazol-7-yl |
 |
25B-NMe7Bim | N-[(benzimidazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-62-6 | 2,5-dimethoxy-4-bromo | H | benzimidazol-7-yl |
 |
FECIMBI-36 | N-[(2-fluoroethoxy)benzyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 2,5-dimethoxy-4-bromo | H | 2-(2-fluoroethoxy)phenyl | |
 |
DOB-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane | 2,5-dimethoxy-4-bromo | methyl | 2-methoxyphenyl | |
 |
25C-NB3OMe | N-(3-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1566571-34-3 | 2,5-dimethoxy-4-chloro | H | 3-methoxyphenyl |
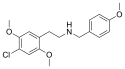 |
25C-NB4OMe | N-(4-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1566571-35-4 | 2,5-dimethoxy-4-chloro | H | 4-methoxyphenyl |
 |
C30-NBOMe | N-(3,4,5-trimethoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1445574-98-0 | 2,5-dimethoxy-4-chloro | H | 3,4,5-trimethoxyphenyl |
 |
25C-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1539266-21-1 | 2,5-dimethoxy-4-chloro | H | 2-fluorophenyl |
 |
25C-NBCl | N-(2-chlorobenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-chlorophenyl | |
 |
25C-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1391488-16-6 | 2,5-dimethoxy-4-chloro | H | 2-hydroxyphenyl |
 |
25C-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1227608-02-7 | 2,5-dimethoxy-4-chloro | H | 2-methoxyphenyl |
 |
25C-NBOEt | N-(2-ethoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-ethoxyphenyl | |
 |
25C-NBOiPr | N-(2-isopropoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-isopropoxyphenyl | |
 |
25F-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-fluorophenyl)-2-aminoethane | 1373917-84-0 | 2,5-dimethoxy-4-fluoro | H | 2-methoxyphenyl |
 |
25CN-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-cyanophenyl)-2-aminoethane | 1539266-32-4 | 2,5-dimethoxy-4-cyano | H | 2-hydroxyphenyl |
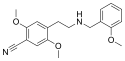 |
25CN-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-cyanophenyl)-2-aminoethane | 1354632-16-8 | 2,5-dimethoxy-4-cyano | H | 2-methoxyphenyl |
 |
25D-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1354632-02-2 | 2,5-dimethoxy-4-methyl | H | 2-methoxyphenyl |
 |
25D-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1391488-44-0 | 2,5-dimethoxy-4-methyl | H | 2-hydroxyphenyl |
 |
25E-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-ethylphenyl)-2-aminoethane | 1354632-14-6 | 2,5-dimethoxy-4-ethyl | H | 2-methoxyphenyl |
 |
25E-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-ethylphenyl)-2-aminoethane | 1391489-79-4 | 2,5-dimethoxy-4-ethyl | H | 2-hydroxyphenyl |
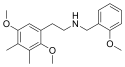 |
25G-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-3,4-dimethylphenyl)-2-aminoethane | 1354632-65-7 | 2,5-dimethoxy-3,4-dimethyl | H | 2-methoxyphenyl |
 |
25H-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxyphenyl)-2-aminoethane | 1566571-52-5 | 2,5-dimethoxy | H | 2-methoxyphenyl |
 |
25I-NB34MD | N-(3,4-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391497-81-6 | 2,5-dimethoxy-4-iodo | H | 3,4-methylenedioxyphenyl |
 |
25I-NB3OMe | N-(3-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1566571-40-1 | 2,5-dimethoxy-4-iodo | H | 3-methoxyphenyl |
 |
25I-NB4OMe | N-(4-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1566571-41-2 | 2,5-dimethoxy-4-iodo | H | 4-methoxyphenyl |
 |
25I-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-21-0 | 2,5-dimethoxy-4-iodo | H | 2-fluorophenyl |
 |
25I-NBBr | N-(2-bromobenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1648649-98-2 | 2,5-dimethoxy-4-iodo | H | 2-bromophenyl |
 |
25I-NBTFM | N-[2-(trifluoromethyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 2-(trifluoromethyl)phenyl | |
 |
25I-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-25-4 | 2,5-dimethoxy-4-iodo | H | 2,3-methylenedioxyphenyl |
 |
25B-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1354632-19-1 | 2,5-dimethoxy-4-bromo | H | 2,3-methylenedioxyphenyl |
 |
25C-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1373879-26-5 | 2,5-dimethoxy-4-chloro | H | 2,3-methylenedioxyphenyl |
 |
25D-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1391488-97-3 | 2,5-dimethoxy-4-methyl | H | 2,3-methylenedioxyphenyl |
 |
25I-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-20-9 | 2,5-dimethoxy-4-iodo | H | 2-hydroxyphenyl |
 |
25I-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-19-6 | 2,5-dimethoxy-4-iodo | H | 2-methoxyphenyl |
 |
DOI-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane | 2,5-dimethoxy-4-iodo | methyl | 2-methoxyphenyl | |
 |
25I-NBMeOH | N-[2-(hydroxymethyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391494-71-5 | 2,5-dimethoxy-4-iodo | H | 2-(hydroxymethyl)phenyl |
 |
25I-NBAm | N-[2-(carbamoyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391494-85-1 | 2,5-dimethoxy-4-iodo | H | 2-(carbamoyl)phenyl |
 |
25I-NMe7DHBF | N-[(2,3-dihydrobenzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 2,3-dihydrobenzofuran-7-yl | |
 |
25I-N2Nap1OH | N-[(1-hydroxynaphthalen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 1-hydroxynaphthalen-2-yl | |
 |
25I-N3MT2M | N-[(3-methoxythiophen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1354632-66-8 | 2,5-dimethoxy-4-iodo | H | 3-methoxythiophen-2-yl |
 |
25I-N4MT3M | N-[(4-methoxythiophen-3-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1354632-73-7 | 2,5-dimethoxy-4-iodo | H | 4-methoxythiophen-3-yl |
 |
25iP-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-isopropylphenyl)-2-aminoethane | 1391487-83-4 | 2,5-dimethoxy-4-isopropyl | H | 2-methoxyphenyl |
 |
25N-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 1354632-03-3 | 2,5-dimethoxy-4-nitro | H | 2-methoxyphenyl |
 |
25N-NBOEt [37] | N-(2-ethoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-ethoxyphenyl | |
 |
25N-NB-2-OH-3-Me | N-(2-hydroxy-3-methylbenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-hydroxy-3-methylphenyl | |
 |
25N-NBOCF2H | N-(2-difluoromethoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-difluoromethoxyphenyl | |
 |
25N-NBPh[38] | N-[(2-phenyl)benzyl]-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | o-biphenyl | |
 |
25N-N1-Nap | N-[(naphthalen-1-yl)methyl]-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 1-naphthyl | |
 |
25P-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-propylphenyl)-2-aminoethane | 1391489-07-8 | 2,5-dimethoxy-4-propyl | H | 2-methoxyphenyl |
 |
25P-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-propylphenyl)-2-aminoethane | 1391490-34-8 | 2,5-dimethoxy-4-propyl | H | 2-hydroxyphenyl |
 |
25TFM-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(trifluoromethyl)phenyl]-2-aminoethane | 1027161-33-6 | 2,5-dimethoxy-4-(trifluoromethyl) | H | 2-methoxyphenyl |
 |
25O-NBcP | N-(2-cyclopropylbenzyl)-1-(2,4,5-trimethoxyphenyl)-2-aminoethane | 2,4,5-trimethoxy | H | 2-cyclopropylphenyl | |
 |
25T-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(methylthio)phenyl]-2-aminoethane | 1539266-47-1 | 2,5-dimethoxy-4-(methylthio) | H | 2-methoxyphenyl |
 |
25T2-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(ethylthio)phenyl]-2-aminoethane | 1539266-51-7 | 2,5-dimethoxy-4-(ethylthio) | H | 2-methoxyphenyl |
 |
25T4-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(isopropylthio)phenyl]-2-aminoethane | 1354632-17-9 | 2,5-dimethoxy-4-(isopropylthio) | H | 2-methoxyphenyl |
 |
25T7-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(propylthio)phenyl]-2-aminoethane | 1539266-55-1 | 2,5-dimethoxy-4-(propylthio) | H | 2-methoxyphenyl |
 |
25T7-NBOH | N-(2-hydroxybenzyl)-1-[2,5-dimethoxy-4-(propylthio)phenyl]-2-aminoethane | 1354632-41-9 | 2,5-dimethoxy-4-(propylthio) | H | 2-hydroxyphenyl |
 |
25AM-NBOMe [39] | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-pentylphenyl]-2-aminoethane | 2,5-dimethoxy-4-(n-pentyl) | H | 2-methoxyphenyl | |
 |
NBOMe-mescaline | N-(2-methoxybenzyl)-1-(3,4,5-trimethoxyphenyl)-2-aminoethane | 1354632-01-1 | 3,4,5-trimethoxy | H | 2-methoxyphenyl |
 |
NBOMe-escaline | N-(2-methoxybenzyl)-1-(3,5-dimethoxy-4-ethoxyphenyl)-2-aminoethane | 3,5-dimethoxy-4-ethoxy | H | 2-methoxyphenyl | |
 |
NBOMe-thiobuscaline | N-(2-methoxybenzyl)-1-(3,5-dimethoxy-4-butylthiophenyl)-2-aminoethane | 3,5-dimethoxy-4-(n-butylthio) | H | 2-methoxyphenyl | |
 |
MDPEA-NBOMe | N-(2-methoxybenzyl)-1-(3,4-methylenedioxyphenyl)-2-aminoethane | 3,4-methylenedioxy | H | 2-methoxyphenyl | |
 |
2C2-NBOMe | N-(2-methoxybenzyl)-1-(2-methoxy-4,5-methylenedioxyphenyl)-2-aminoethane | 2-methoxy-4,5-methylenedioxy | H | 2-methoxyphenyl | |
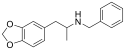 |
MDBZ | N-benzyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane | 65033-29-6 | 3,4-methylenedioxy | methyl | phenyl |
 |
Clobenzorex | N-(2-chlorobenzyl)-1-phenyl-2-aminopropane | 13364-32-4 | H | methyl | 2-chlorophenyl |
 |
4-EA-NBOMe | N-(2-methoxybenzyl)-1-(4-ethylphenyl)-2-aminopropane | 4-ethyl | methyl | 2-methoxyphenyl | |
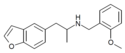 |
5-APB-NBOMe | N-(2-methoxybenzyl)-1-(benzofuran-5-yl)-2-aminopropane | benzofuran-5-yl instead of phenyl | methyl | 2-methoxyphenyl |
Similar compounds with related structures are also known including;
| Chemical structure | Common name | Chemical name | CAS number |
|---|---|---|---|
 |
25B-N1POMe | N-[1-(2-methoxyphenyl)ethyl]-2,5-dimethoxy-4-bromophenethylamine | 1335331-49-1 (R) 1335331-51-5 (S) |
 |
2C-B-AN [40][41] | 2-phenyl-2-[2-(2,5-dimethoxy-4-bromophenyl)ethylamino]acetonitrile | |
 |
25B-N(BOMe)2 | 2-(4-Bromo-2,5-dimethoxyphenyl)-N,N-bis(2-methoxybenzyl)ethan-1-amine | |
 |
25CN-N3DHBF[42] | 4-(2-[(2,3-dihydro-1-benzofuran-3-yl)amino]ethyl)-2,5-dimethoxybenzonitrile | |
 |
2CBCB-NBOMe | N-[(3-bromo-2,5-dimethoxy-bicyclo[4,2,0]octa-1,3,5-trien-7-yl)methyl]-1-(2-methoxyphenyl)methanamine | 1354634-09-5 |
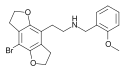 |
2CBFly-NBOMe | N-(2-methoxybenzyl)-1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | 1335331-42-4 |
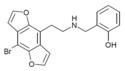 |
2C-B-DRAGONFLY-NBOH | N-(2-hydroxybenzyl)-1-(8-bromobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | 1335331-45-7 |
 |
2C-B-FLY-NB2EtO5Cl [43] | N-(2-ethoxy-5-chlorobenzyl)-1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | |
 |
DMBMPP | (S,S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine | 1391499-52-7 |
 |
25B-NAcPip | 2-{[2-(4-bromo-2,5-dimethoxyphenyl)ethyl]amino}-1-(piperidin-1-yl)ethanone | |
 |
ZDCM-04 | 1,3-dimethyl-7-{2-[1-(2,5-dimethoxy-4-chlorophenyl)propan-2-ylamino]ethyl}purine-2,6-dione | |
 |
RH-34 | 3-[2-(2-methoxybenzylamino)ethyl]-1H-quinazoline-2,4-dione | 1028307-48-3 |
 |
5-MeO-T-NBOMe[44] | N-(2-methoxybenzyl)-5-methoxytryptamine | 1335331-37-7 |
 |
5MT-NB3OMe | N-(3-methoxybenzyl)-5-methoxytryptamine | 1648553-42-7 |
A large number of substances in the 25-NB class are Class A drugs in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971[45] or are otherwise covered by the Psychoactive Substances Act 2016.[46]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.