2C (psychedelics)
Family of phenethylamine psychedelics From Wikipedia, the free encyclopedia
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring.[1][2][3] Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds.[4]

Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL (Phenethylamines i Have Known And Loved).[3] Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.[5][1][3] 2C-B is the most popular of the 2C drugs.[3]
Use
Summarize
Perspective
The 2C drugs are orally active, are used at oral doses of 6 to 150 mg depending on the drug, and have durations of 3 to 48 hours depending on the drug.[1][6][5][7] However, many have doses in the range of 10 to 60 mg and durations in the range of 4 to 12 hours.[1] The 2C drugs produce psychedelic effects.[1][5][8][3] Some, such as 2C-B, have also been reported to have some entactogenic qualities, though findings appear to be mixed.[8][3][9][10]
| Compound | Chemical name | Dosage | Duration | |
|---|---|---|---|---|
| 2C-AL | 4-Allyl-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-B | 4-Bromo-2,5-dimethoxyphenethylamine | 10–35 mg | 4–8 hours | |
| 2C-Bu | 4-Butyl-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-C | 4-Chloro-2,5-dimethoxyphenethylamine | 20–40 mg | 4–8 hours | |
| 2C-CN | 4-Cyano-2,5-dimethoxyphenethylamine | >22 mg | Unknown | |
| 2C-CP | 4-Cyclopropyl-2,5-dimethoxyphenethylamine | 15–35 mg | 3–6 hours | |
| 2C-D | 4-Methyl-2,5-dimethoxyphenethylamine | 20–60 mg | 4–6 hours | |
| 2C-E | 4-Ethyl-2,5-dimethoxyphenethylamine | 10–25 mg | 6–12 hours | |
| 2C-EF | 4-Fluoroethyl-2,5-dimethoxyphenethylamine | 10–25 mg | Unknown | |
| 2C-F | 4-Fluoro-2,5-dimethoxyphenethylamine | ≥250 mg | Unknown | |
| 2C-G | 3,4-Dimethyl-2,5-dimethoxyphenethylamine | 20–35 mg | 18–30 hours | |
| 2C-G-3 | 3,4-Trimethylene-2,5-dimethoxyphenethylamine | 16–25 mg | 12–24 hours | |
| 2C-G-5 | 3,4-Norbornyl-2,5-dimethoxyphenethylamine | 10–16 mg | 32–48 hours | |
| 2C-H | 2,5-Dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-I | 4-Iodo-2,5-dimethoxyphenethylamine | 14–22 mg | 6–10 hours | |
| 2C-iBu | 4-Isobutyl-2,5-dimethoxyphenethylamine | ≥5 mg | ~20 hours | |
| 2C-iP | 4-Isopropyl-2,5-dimethoxyphenethylamine | 8–25 mg | 8–12 hours | |
| 2C-N | 4-Nitro-2,5-dimethoxyphenethylamine | 100–150 mg | 4–6 hours | |
| 2C-O | 4-Methoxy-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-O-4 | 4-Isopropoxy-2,5-dimethoxyphenethylamine | >60 mg | Unknown | |
| 2C-O-22 | 4-(2,2,2-Trifluoroethoxy)-2,5-dimethoxyphenethylamine | ≥57 mg | Unknown | |
| 2C-P | 4-Propyl-2,5-dimethoxyphenethylamine | 6–10 mg | 5–16 hours | |
| 2C-Ph (2C-BI-1) | 4-Phenyl-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-SE | 4-Methylseleno-2,5-dimethoxyphenethylamine | ~100 mg | 6–8 hours | |
| 2C-T (2C-T-1) | 4-Methylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 3–5 hours | |
| 2C-T-2 | 4-Ethylthio-2,5-dimethoxyphenethylamine | 12–25 mg | 6–8 hours | |
| 2C-T-3 (2C-T-20) | 4-Methallylthio-2,5-dimethoxyphenethylamine | 15–40 mg | 8–14 hours | |
| 2C-T-4 | 4-Isopropylthio-2,5-dimethoxyphenethylamine | 8–20 mg | 12–18 hours | |
| 2C-T-7 | 4-Propylthio-2,5-dimethoxyphenethylamine | 10–30 mg | 8–15 hours | |
| 2C-T-8 | 4-Cyclopropylmethylthio-2,5-dimethoxyphenethylamine | 30–50 mg | 10–15 hours | |
| 2C-T-9 | 4-tert-Butylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 12–18 hours | |
| 2C-T-13 | 4-(2-Methoxyethylthio)-2,5-dimethoxyphenethylamine | 25–40 mg | 6–8 hours | |
| 2C-T-15 | 4-Cyclopropylthio-2,5-dimethoxyphenethylamine | >30 mg | Several hours | |
| 2C-T-16 | 4-Allylthio-2,5-dimethoxyphenethylamine | 10–25 mg | 4–6 hours | |
| 2C-T-17 | 4-sec-Butylthio-2,5-dimethoxyphenethylamine | 60–100 mg | 10–15 hours | |
| 2C-T-19 | 4-Butylthio-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-T-21 | 4-(2-Fluoroethylthio)-2,5-dimethoxyphenethylamine | 8–20 mg | 7–10 hours | |
| 2C-T-21.5 | 4-(2,2-Difluoroethylthio)-2,5-dimethoxyphenethylamine | 12–30 mg | 8–14 hours | |
| 2C-T-22 | 4-(2,2,2-Trifluoroethylthio)-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-T-25 | 4-Isobutylthio-2,5-dimethoxyphenethylamine | >30 mg | Unknown | |
| 2C-T-27 | 4-Benzylthio-2,5-dimethoxyphenethylamine | ≥80 mg | Unknown | |
| 2C-T-28 | 4-(3-Fluoropropylthio)-2,5-dimethoxyphenethylamine | 8–20 mg | 8–10 hours | |
| 2C-T-30 | 4-(4-Fluorobutylthio)-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-T-33 | 4-(3-Methoxybenzylthio)-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-T-36 (2C-T-TFM) | 4-Trifluoromethylthio-2,5-dimethoxyphenethylamine | Unknown | Unknown | |
| 2C-tBu | 4-tert-Butyl-2,5-dimethoxyphenethylamine | >5–10 mg | Unknown | |
| 2C-TFE | 4-(2,2,2-Trifluoroethyl)-2,5-dimethoxyphenethylamine | 5–15 mg | 12–24 hours | |
| 2C-TFM | 4-Trifluoromethyl-2,5-dimethoxyphenethylamine | 3–6 mg | ≥5–10 hours | |
| 2C-V | 4-Ethenyl-2,5-dimethoxyphenethylamine | ~25 mg | ~5 hours | |
| 2C-YN | 4-Ethynyl-2,5-dimethoxyphenethylamine | ~50 mg | ~2 hours | |
| Refs: [1][6][3][7][5][2][11][12][13][4] | ||||
Pharmacology
Summarize
Perspective
Pharmacodynamics
Actions
The 2C drugs act as agonists of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.[14][15][16][17][18] They are partial agonists of the serotonin 5-HT2A receptor.[14][15] Most of the 2C drugs have much lower affinity for the serotonin 5-HT1A receptor than for the serotonin 5-HT2A receptor.[14][15][16][17] Most of the 2C drugs have also shown about 5- to 15-fold higher affinity for the serotonin 5-HT2A receptor over the serotonin 5-HT2C receptor and about 15- to 100-fold higher affinity for the serotonin 5-HT2A receptor over the serotonin 5-HT1A receptor.[15] The psychedelic effects of the 2C drugs are thought to be mediated specifically by activation of the serotonin 5-HT2A receptor.[14][16][18]
Unlike many other phenethylamines, 2C drugs, including 2C-C, 2C-D, 2C-E, 2C-I, and 2C-T-2 among others, are inactive as monoamine releasing agents and reuptake inhibitors.[14][19][16][15][18] Most of the 2C drugs are agonists of the rat and mouse trace amine-associated receptor 1 (TAAR1).[14][20][21][15] However, most are inactive as agonists of the human TAAR1.[14][20][21][15] The 2C drugs show very weak monoamine oxidase inhibition, including of monoamine oxidase A (MAO-A) and/or monoamine oxidase B (MAO-B).[14]
| Drug | 5-HT1A | 5-HT1B | 5-HT2A | 5-HT2B | 5-HT2C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki (nM) | EC50 (nM) | Emax (%) | Ki (nM) | Ki (nM) | EC50 (nM) | Emax (%) | Ki (nM) | EC50 (nM) | Emax (%) | Ki (nM) | EC50 (nM) | Emax (%) | |
| 2C-B | 240–311 | ND | ND | 104.4 | 6.9–27.6 | 1.89–80 | 5–99% | 13.5 | 75–130 | 52–89% | 43–89.5 | 0.031–0.264 | 104–116% |
| 2C-C | 190–740 | >10,000 | <25% | 252.9 | 5.47–13 | 9.27–200 | 49–102% | ND | 280 | 81% | 5.4–90 | 24.2 | 94% |
| 2C-D | 440–1,630 | >10,000 | <25% | ND | 23.9–32.4 | 43.5–350 | 41–125% | ND | 230 | 77% | 12.7–150 | 71.1 | 100% |
| 2C-E | 307.3–1,190 | >10,000 | <25% | ND | 4.50–43.9 | 2.5–110 | 40–125% | 25.1 | 190 | 66% | 5.4–104.1 | 0.233–18.0 | 98–106% |
| 2C-H | 70 | ND | ND | ND | 1,600 | 2,408–9,400 | 28–67% | ND | 6,200 | 46% | 4,100 | ND | ND |
| 2C-I | 180–970 | 4,900 | 102% | ND | 3.5–9.3 | 3.83–60 | 15–82% | ND | 150 | 70% | 10.2–40 | 2.8 | 79–100% |
| 2C-N | 2,200 | ND | ND | ND | 23.5 | 170 | 20–48% | ND | 730 | 74% | 370 | ND | 40–50% |
| 2C-P | 110 | ND | ND | ND | 8.1 | 90 | 63% | ND | 130 | 72% | 40 | ND | ND |
| 2C-T-1 | 1,035 | ND | ND | ND | 49 | 2.0 | 75% | ND | 57 | 58% | 347 | ND | ND |
| 2C-T-2 | 370–1,740 | 3,000 | 76% | 857.5 | 9–39.9 | 0.354–80 | 67–128% | 6 | 130 | 75% | 14.2–69 | 0.0233–3.8 | 87–107% |
| 2C-T-4 | 470–916 | ND | ND | ND | 27.9–54 | 5.5–220 | 56–87% | ND | 63–160 | 68–75% | 180–295 | ND | ND |
| 2C-T-7 | 520–878 | ND | ND | ND | 5.3–6.5 | 1.2–130 | 49–101% | ND | 52–350 | 45–75% | 39–54 | ND | ND |
| Notes: The smaller the value, the more avidly the drug binds to or activates the site. Refs: [15][16][17][14][22][23][24] | |||||||||||||
Effects
In accordance with their psychedelic effects in humans, the 2C drugs produce the head-twitch response and wet dog shakes, behavioral proxies of psychedelic effects, in rodents.[14] At least some 2C drugs, such as 2C-D and 2C-E, produce hyperlocomotion at lower doses in rodents.[14] All 2C drugs produce hypolocomotion at higher doses in rodents.[14] 2C drugs, including 2C-C, 2C-D, 2C-E, and 2C-I, substitute partially to fully for psychedelics like DOM, DMT, and LSD and/or for the entactogen MDMA in rodent drug discrimination tests.[14][16] However, none of the assessed 2C drugs substituted for dextromethamphetamine, suggesting that they lack amphetamine-type or stimulant-like effects.[14][16]
In contrast to most psychedelics, at least two assessed 2C drugs, 2C-C and 2C-P, have shown reinforcing effects in rodents, including conditioned place preference (CPP) and self-administration.[14][25] The mechanism by which these effects are mediated is unknown.[14] However, it may be related to reduced expression of the dopamine transporter (DAT) and increased DAT phosphorylation, in turn resulting in increased extracellular dopamine levels in certain brain areas.[14][25] These 2C drugs might have misuse potential in humans.[14][25] Similar reinforcing effects in animals have been observed for NBOMe analogues of 2C drugs, including 25B-NBOMe, 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, and 25N-NBOMe.[14][26][27][28][29][30][31]
Similarly to DOI, tolerance has been found to gradually develop to the head-twitch response induced by 2C-T-7 with chronic administration in rodents.[14]
Various 2C drugs show potent anti-inflammatory effects mediated by serotonin 5-HT2A receptor activation.[32] Among these include 2C-I, 2C-B, 2C-H, and 2C-iBu.[32][33] Others, such as 2C-B-Fly and 2C-T-33, were less effective.[32] 2C-iBu has shown a greater separation between anti-inflammatory effects and psychedelic-like effects in animals than other 2C drugs and is being investigated for possible use as a pharmaceutical drug.[33][34]
Pharmacokinetics
The 2C drugs are orally active.[1] They are metabolized by O-demethylation and deamination.[1][35] This is mediated specifically by monoamine oxidase (MAO) enzymes MAO-A and MAO-B, whereas cytochrome P450 enzymes appear to metabolize only some 2C drugs and to have only a very small role.[35]
Interactions
The 2C drugs are metabolized by the monoamine oxidase (MAO) enzymes, including both MAO-A and MAO-B.[1][35] As a result, they may be potentiated by monoamine oxidase inhibitors (MAOIs), such as phenelzine, tranylcypromine, moclobemide, and selegiline.[1][35][36] This may lead to overdose and serious toxicity.[1][35][36]
History
Summarize
Perspective
2,4,5-Trimethoxyphenethylamine (2,4,5-TMPEA; 2C-O or "2C-MeO") was first synthesized by Jansen and was found to produce psychedelic effects similar to those of mescaline (3,4,5-trimethoxyphenethylamine).[37][38] He published his findings in 1931.[37][38] However, subsequent studies in the 1960s and 1970s suggested that 2,4,5-TMPEA may actually be inactive as a psychedelic in animals and humans.[37]
2C-D (2C-M) was the first of the 2C drugs besides 2C-O to be discovered.[2][39][40][41] It was synthesized and studied in animals by Ho and colleagues and they published their findings in 1970.[2][39][40][41] Alexander Shulgin synthesized 2C-B and 2C-D in 1974 and discovered their psychedelic effects in self-experiments conducted in 1974 and 1975.[1][42][2][39][43] He published his findings in the scientific literature in 1975.[1][42][2][39][43] 2C-T was first described by Shulgin and David E. Nichols in 1976.[44] 2C-I was first described by Shulgin and colleagues in 1977 and initial psychoactivity was reported by Shulgin in 1978.[37][45] Shulgin also first synthesized 2C-E in 1977.[46][47] Subsequently, numerous other 2C drugs have been synthesized and characterized.[5][6][2][1][42]
2C-B gained popularity as a recreational drug and MDMA alternative in the mid-1980s and became a controlled substance in the United States in 1994.[1][3] It is said to be the most popular of the 2C drugs.[3]
Legal status
Canada
As of October 12, 2016, the 2C-x family of substituted phenethylamines is a controlled substance (Schedule III) in Canada.[48]
List of 2C drugs
| Name | R3 | R4 | Structure | CAS # |
|---|---|---|---|---|
| 2C-B | H | Br | 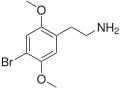 |
66142-81-2 |
| 2C-Bn | H | CH2C6H5 |  |
|
| 2C-Bu | H | CH2CH2CH2CH3 |  |
|
| 2C-C | H | Cl | 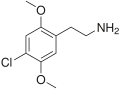 |
88441-14-9 |
| 2C-C-3 [49] | Cl | Cl |  |
|
| 2C-CN | H | C≡N |  |
88441-07-0 |
| 2C-D | H | CH3 |  |
24333-19-5 |
| 2C-E | H | CH2CH3 |  |
71539-34-9 |
| 2C-EF | H | CH2CH2F |  |
1222814-77-8 |
| 2C-F | H | F | 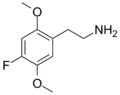 |
207740-15-6 |
| 2C-G | CH3 | CH3 | 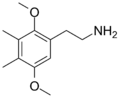 |
207740-18-9 |
| 2C-G-1 | CH2 |  |
||
| 2C-G-2 | (CH2)2 |  |
||
| 2C-G-3 | (CH2)3 |  |
207740-19-0 | |
| 2C-G-4 | (CH2)4 | 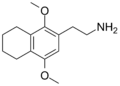 |
952006-59-6 | |
| 2C-G-5 | (CH2)5 |  |
207740-20-3 | |
| 2C-G-6 | (CH2)6 |  |
||
| 2C-G-N | (CH)4 |  |
207740-21-4 | |
| 2C-H | H | H |  |
3600-86-0 |
| 2C-I | H | I |  |
69587-11-7 |
| 2C-iBu | H | iBu |  |
|
| 2C-iP | H | CH(CH3)2 |  |
1498978-47-4 |
| 2C-tBu | H | C(CH3)3 |  |
|
| 2C-CP | H | C3H5 |  |
2888537-46-8 |
| 2C-CPE | H | C5H9 | 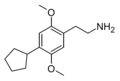 |
|
| 2C-N | H | NO2 | 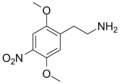 |
261789-00-8 |
| 2C-NH2 | H | NH2 | 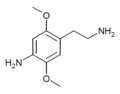 |
168699-66-9 |
| 2C-PYR | H | Pyrrolidine |  |
|
| 2C-PIP | H | Piperidine | 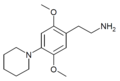 |
|
| 2C-O | H | OCH3 |  |
15394-83-9 |
| 2C-O-4 | H | OCH(CH3)2 | 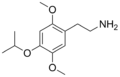 |
952006-65-4 |
| 2C-MOM [50] | H | CH2OCH3 |  |
|
| 2C-P | H | CH2CH2CH3 |  |
207740-22-5 |
| 2C-Ph (2C-BI-1) | H | C6H5 | 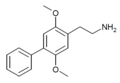 |
|
| 2C-Se | H | Se CH3 | 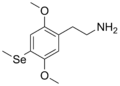 |
1189246-68-1 |
| 2C-T | H | SCH3 |  |
61638-09-3 |
| 2C-T-2 | H | SCH2CH3 | 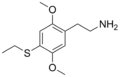 |
207740-24-7 |
| 2C-T-3[51] | H | SCH2C(=CH2)CH3 |  |
648957-40-8 |
| 2C-T-4 | H | SCH(CH3)2 | 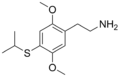 |
207740-25-8 |
| 2C-T-5[51] |  |
|||
| 2C-T-6[51] |  |
|||
| 2C-T-7 | H | S(CH2)2CH3 |  |
207740-26-9 |
| 2C-T-8 | H | SCH2CH(CH2)2 | 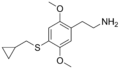 |
207740-27-0 |
| 2C-T-9[51] |  |
207740-28-1 | ||
| 2C-T-10[51] |  |
|||
| 2C-T-11[51] |  |
|||
| 2C-T-12[51] |  |
|||
| 2C-T-13 | H | S(CH2)2OCH3 |  |
207740-30-5 |
| 2C-T-14[51] |  |
|||
| 2C-T-15 | H | SCH(CH2)2 | 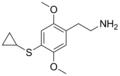 |
|
| 2C-T-16[52] | H | SCH2CH=CH2 |  |
648957-42-0 |
| 2C-T-17 | H | SCH(CH3)CH2CH3 |  |
207740-32-7 |
| 2C-T-18[51] |  |
|||
| 2C-T-19 | H | SCH2CH2CH2CH3 |  |
|
| 2C-T-21 | H | S(CH2)2F |  |
207740-33-8 |
| 2C-T-21.5[51] |  |
648957-46-4 | ||
| 2C-T-22[51] | 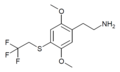 |
648957-48-6 | ||
| 2C-T-23[51] | 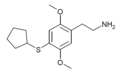 |
|||
| 2C-T-24[51] |  |
|||
| 2C-T-25[51] |  |
|||
| 2C-T-27[51] |  |
648957-52-2 | ||
| 2C-T-28[51] |  |
648957-54-4 | ||
| 2C-T-30[51] | 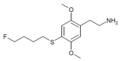 |
|||
| 2C-T-31[51] |  |
|||
| 2C-T-32[51] |  |
|||
| 2C-T-33[51] |  |
|||
| 2C-T-DFM | H | SCF2H |  |
|
| CYB210010 (2C-T-TFM)[53] | H | SCF3 | 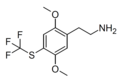 |
|
| 2C-T-DFP | H | SCH2CH2CF2H |  |
|
| 2C-T-PARGY | H | SCH2C≡CH | 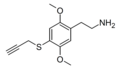 |
|
| 2C-DFM [4]: 770 | H | CHF2 |  |
|
| 2C-TFM | H | CF3 |  |
159277-08-4 |
| 2C-TFE | H | CH2CF3 |  |
|
| 2C-PFE | H | CF2CF3 | 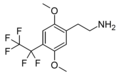 |
|
| 2C-PFS | H | SF5 |  |
|
| 2C-YN | H | C≡CH |  |
752982-24-4 |
| 2C-V | H | CH=CH2 | 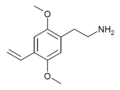 |
|
| 2C-AL[54] | H | CH2CH=CH2 |  |
|
Related compounds
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
