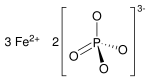
磷酸亞鐵是磷酸的鐵鹽,化學式Fe3(PO4)2。它在自然界中以藍鐵礦形式存在[7],可用作肥料。[8]
| 磷酸亞鐵 | |
|---|---|
| |

| |
| IUPAC名 Iron(II) phosphate | |
| 別名 | 磷酸鐵(II) |
| 識別 | |
| CAS號 | 14940-41-1 |
| PubChem | 9863567 |
| ChemSpider | 8039263 |
| SMILES |
|
| 性質 | |
| 化學式 | Fe3O8P2 |
| 摩爾質量 | 357.48 g·mol−1 |
| 外觀 | 無色固體[1] 藍灰色晶體(八水)[2] |
| 密度 | 3.94 g/cm3[3] 2.58 g/cm3(八水合物)[4] |
| 熔點 | 180 °C(453 K)((八水合物)分解[6]) |
| 溶解性(水) | 不溶(八水)[5] |
| 溶解性 | 不溶於乙醇,可溶於酸(八水)[5] |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
製備
- 3 FeSO4 + 2 Na3PO4 → Fe3(PO4)2↓ + 3 Na2SO4
磷酸和鐵直接反應只會得到Fe(H2PO4)2·2H2O[1]。無水磷酸亞鐵可以由硝酸鐵和磷酸二氫銨反應,然後加熱產生的棕色物質而成。[7]
物理性質
無水磷酸亞鐵呈單斜晶系,空間群P21/c (No. 14)。[3]其四水合物和八水合物的晶體結構也是單斜晶系,空間群分別為P21/a (No. 14)和C2/m (No. 12)。[9][10][11]
剛沉澱產生的八水合磷酸亞鐵是藍灰色固體,在空氣氧化下轉變成靛藍色。對其進一步氧化會破壞其晶體結構,產生黃棕色的無定形體。[8]
化學性質
- 水合物加熱分解:
磷酸亞鐵可以和N2H4形成各種配合物,如深綠色的Fe3(PO4)2·3N2H4·5H2O[12]、白色的Fe3(PO4)2·6N2H4·2.75H2O、[13]淺綠色的Fe3(PO4)2·6N2H4·8H2O[14]以及白色的Fe3(PO4)2·7N2H4·nH2O。[12]它們都會爆炸。
參見
參考
延伸閱讀
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.



![{\displaystyle {\mathsf {Fe_{3}(PO_{4})_{2}\cdot 8H_{2}O\ {\xrightarrow[{-H_{2}O}]{T}}\ Fe_{3}(PO_{4})_{2}\cdot 4H_{2}O\ {\xrightarrow[{-H_{2}O}]{180^{o}C}}\ Fe_{3}(PO_{4})_{2}}}}](http://wikimedia.org/api/rest_v1/media/math/render/svg/530df214323b0dc99e2019e7d43914c2b620eba9)
