ウィキペディアから
ABL1は、ヒトでは9番染色体に位置するABL1遺伝子(以前のシンボルはABL)によってコードされているタンパク質である[5]。哺乳類ゲノムに存在するホモログを表す場合にはc-Abl、ウイルスの場合にはv-Ablという表記が用いられることがあり、当初エーベルソンマウス白血病ウイルス(Abelson murine leukemia virus)から単離されたことに由来する[6]。
ABL1がん原遺伝子は細胞質と核に位置するチロシンキナーゼをコードし、細胞分化、細胞分裂、細胞接着、そしてDNA修復などのストレス応答といった過程への関与が示唆されている[7][8][9][10]。ABL1タンパク質の活性はSH3ドメインによって負に調節されており、SH3ドメイン領域が欠失することでABL1はがん遺伝子となる。t(9;22)転座はBCR遺伝子とABL1遺伝子とのhead-to-tail型の融合を引き起こし、慢性骨髄性白血病の多くの症例でみられる融合遺伝子が形成される。普遍的に発現しているABL1チロシンキナーゼのDNA結合活性はCDC2によるリン酸化によって調節され、このことはABL1が細胞周期機能に関与していることを示唆している。ABL1遺伝子は6 kbまたは7 kbのいずれかの長さのmRNA転写産物として発現する。最初のエクソンが選択的エクソンであり、エクソン2–11は共通である[11]。
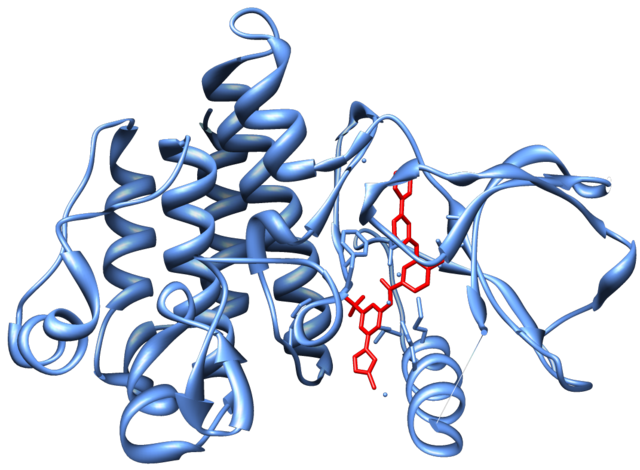
ABL1遺伝子の変異は慢性骨髄性白血病(CML)と関係している。CMLでは、この遺伝子が22番染色体上のBCR遺伝子へ転座することによって活性化されている。この染色体異常はCMLに特徴的であり、稀に他の白血病でもみられる。この新たな融合遺伝子BCR-ABLは調節を受けない細胞質型チロシンキナーゼをコードし、細胞周期調節システムの媒介因子を活性化することで、サイトカインによる調節を受けることのない細胞増殖を可能にし、クローン性骨髄増殖性疾患を引き起こす。BCR-ABLタンパク質はさまざまな低分子によって阻害することができる。そうした阻害薬の1つとしてイマチニブがあり、チロシンキナーゼドメインに結合してBCR-ABLの細胞周期への影響を阻害する。イマチニブ耐性を持つBCR-ABL変異体を阻害する、次世代型BCR-ABLチロシンキナーゼ阻害薬の開発も行われている[12]。
ABL1は次に挙げる因子と相互作用することが示されている。
ABL1の発現がmiR-203によって調節されている証拠も得られている[61]。
Seamless Wikipedia browsing. On steroids.