Mangaan [maŋˈɡaːn] as en cheemisk element mä det sümbool Mn an det atoomnumer 25. Mangaan as en salwerwitj an hard auergungsmetal an liket iisen.
| Eegenskapen | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Algemian | ||||||||||||||||||||||||||||||||||||||||
| Nööm, Symbool, Numer | Mangaan, Mn, 25 | |||||||||||||||||||||||||||||||||||||||
| Seerie | Auergungsmetal | |||||||||||||||||||||||||||||||||||||||
| Skööl, Periode, Blook | 7, 4, d | |||||||||||||||||||||||||||||||||||||||
| Klöör, Skak | salwern metalen | |||||||||||||||||||||||||||||||||||||||
| CAS-Numer | 7439-96-5 | |||||||||||||||||||||||||||||||||||||||
| Uundial | 0,085 %[1] | |||||||||||||||||||||||||||||||||||||||
| Atomaar [2] | ||||||||||||||||||||||||||||||||||||||||
| Atoommase | 54,938049 u | |||||||||||||||||||||||||||||||||||||||
| Atoomraadius (bereegent) | 140 (161) pm | |||||||||||||||||||||||||||||||||||||||
| Kovalent-Raadius | low-spin: 139 pm, high-spin: 161 pm | |||||||||||||||||||||||||||||||||||||||
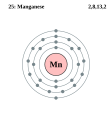
| Elektroonen | [Ar] 3d5 4s2 | |||||||||||||||||||||||||||||||||||||||
| Energii | 4,1 eV[3] | |||||||||||||||||||||||||||||||||||||||
| 1. Ionisiarang | 717,3 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 2. Ionisiarang | 1509 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 3. Ionisiarang | 3248 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 4. Ionisiarang | 4940 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 5. Ionisiarang | 6990 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 6. Ionisiarang | 9220 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| 7. Ionisiarang | 11.500 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| Füsikaalisk [4] | ||||||||||||||||||||||||||||||||||||||||
| Tustant | fääst | |||||||||||||||||||||||||||||||||||||||
| Feranrangen | sjauer | |||||||||||||||||||||||||||||||||||||||
| Sachthaid | 7,43 g/cm3 (25 °C)[5] | |||||||||||||||||||||||||||||||||||||||
| Hardhaid | 6,0 | |||||||||||||||||||||||||||||||||||||||
| Magnetismus | paramagneetisk (: = 9,0 · 10−4; : = 8,2 · 10−4)[6] | |||||||||||||||||||||||||||||||||||||||
| Smoltponkt | 1519 K (1246 °C) | |||||||||||||||||||||||||||||||||||||||
| Köögponkt | 2373 K[7] K (2100 °C) | |||||||||||||||||||||||||||||||||||||||
| Molaar Rüm | 7,35 × 10−6 m3/mol | |||||||||||||||||||||||||||||||||||||||
| Dampwaremk | 225 kJ/mol[7] kJ/mol | |||||||||||||||||||||||||||||||||||||||
| Smoltwaremk | 13,2 kJ/mol | |||||||||||||||||||||||||||||||||||||||
| Faard faan a tuun | 5150 m/s bi 293,15 K | |||||||||||||||||||||||||||||||||||||||
| Waremk | 479,5[1] J/(kg · K) | |||||||||||||||||||||||||||||||||||||||
| Elektrisk struumfeerang | 6,94 × 105 A/(V · m) | |||||||||||||||||||||||||||||||||||||||
| Waremkfeerang | 7,8 W/(m · K) | |||||||||||||||||||||||||||||||||||||||
| Cheemisk [8] | ||||||||||||||||||||||||||||||||||||||||
| Oksidatsionstustant | 1, 2, 3, 4, (5), 6, 7 | |||||||||||||||||||||||||||||||||||||||
| Oksiiden | MnO, MnO2, Mn2O3, Mn2O7, Mn3O4 | |||||||||||||||||||||||||||||||||||||||
| Sür of baasisk | stark sür | |||||||||||||||||||||||||||||||||||||||
| Normoolpotentiaal | −1,18 V (Mn2+ + 2 e− → Mn) | |||||||||||||||||||||||||||||||||||||||
| Elektronegatiwiteet | 1,55 (Pauling-Skala) | |||||||||||||||||||||||||||||||||||||||
| Isotoopen | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| Muar isotoopen bi List faan isotoopen | ||||||||||||||||||||||||||||||||||||||||
| NMR-Eegenskapen | ||||||||||||||||||||||||||||||||||||||||
| Seekerhaid | ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| Miast wurd SI-ianhaiden brükt. | ||||||||||||||||||||||||||||||||||||||||
Bilen
- SIMS spektrum faan a isotoopen
- Elektroonenskel
- Rhodochrosit (ruad) an Manganit (suart).
- Rian Mangaan
Kwelen
Luke uk diar
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
 ...
...