Top Qs
Timeline
Chat
Perspective
Titanium(IV) nitrate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
Remove ads
Remove ads
Preparation
Similarly to its original method,[7][8] Ti(NO3)4 is prepared by the nitration of titanium tetrachloride using dinitrogen pentoxide[9] or chlorine nitrate:[10]
- TiCl4 + 4 N2O5 → Ti(NO3)4 + 4 ClNO2
Hydrated titanium nitrate, the nitrate salt of the aquo complex [Ti(H2O)6]3+, is produced upon dissolution of titanium compounds in nitric acid.[11]
Structure
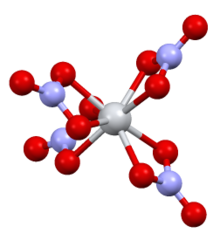
The complex has D2d symmetry, with four bidentate nitrate ligands. The N-O distances are 1·29 Å and 1·185 Å (noncoordinated).[6]
Physical properties
In the infrared spectrum, it absorbs strongly at 1635 cm−1, assigned to a N-O vibrational mode.[12]
It is soluble in nonpolar solvents silicon tetrachloride and carbon tetrachloride.[13][8]
Reactions
Titanium nitrate is hygroscopic, converting to ill-defined hydrates.[14] The anhydrous material is highly reactive, even toward hydrocarbons.[14] Titanium nitrate also reacts with n-dodecane,[15] p-dichlorobenzene, anisole, biphenyl,[15][16]
It decomposes thermally to titanium dioxide.[17]
References
Other reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads