Diammonium phosphate
Chemical compound From Wikipedia, the free encyclopedia
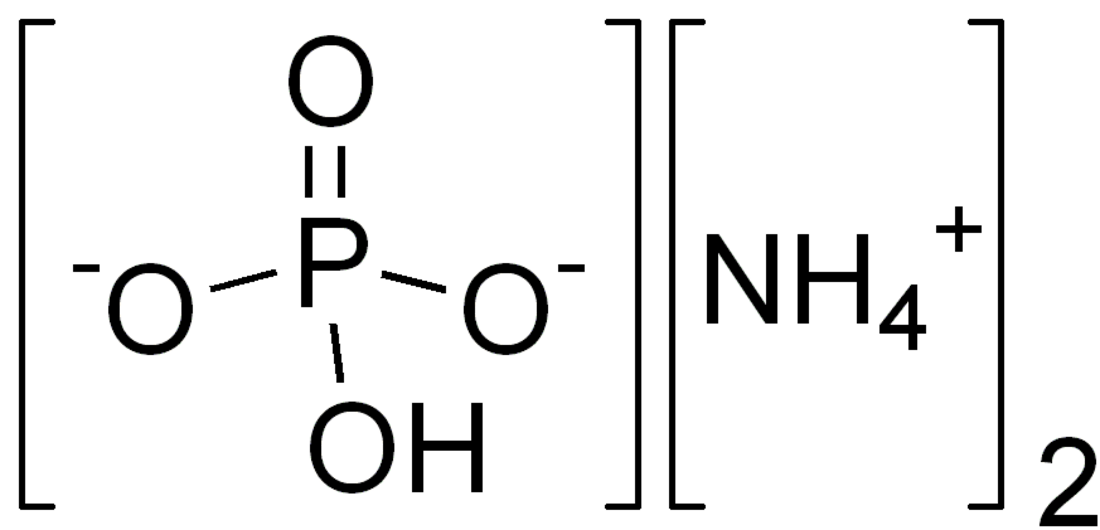
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4)) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.
 | |
| Names | |
|---|---|
| IUPAC name
diammonium hydrogen phosphate | |
| Other names
ammonium monohydrogen phosphate, ammonium hydrogen phosphate, ammonium phosphate dibasic | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.079 |
| E number | E342(ii) (antioxidants, ...) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)2HPO4 | |
| Molar mass | 132.06 g/mol |
| Appearance | colorless monoclinic crystals |
| Density | 1.619 g/cm3 |
| Melting point | 155 °C (311 °F; 428 K) decomposes |
| 57.5 g/100 mL (10 °C) 106.7 g/100 mL (70 °C) | |
| Solubility | insoluble in alcohol, acetone and liquid ammonia |
Refractive index (nD) |
1.52 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−1566.91 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0217 |
| Related compounds | |
Other anions |
Monoammonium phosphate Triammonium phosphate |
Other cations |
Disodium phosphate Dipotassium phosphate |
Related compounds |
Ammonium nitrate Ammonium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Solid diammonium phosphate shows a dissociation pressure of ammonia as given by the following expression and equation:[2]
- (NH4)2HPO4(s) ⇌ NH3(g) + (NH4)H2PO4(s)
At 100 °C, the dissociation pressure of diammonium phosphate is approximately 5 mmHg.[3]
According to the diammonium phosphate MSDS from CF Industries, Inc., decomposition starts as low as 70 °C: "Hazardous Decomposition Products: Gradually loses ammonia when exposed to air at room temperature. Decomposes to ammonia and monoammonium phosphate at around 70 °C (158 °F). At 155 °C (311 °F), DAP emits phosphorus oxides, nitrogen oxides and ammonia."
Uses
Summarize
Perspective
DAP is used as a fertilizer.[4] When applied as plant fertilizer, it temporarily increases the soil pH, but over a long term the treated ground becomes more acidic than before, upon nitrification of the ammonium. It is incompatible with alkaline chemicals because its ammonium ion is more likely to convert to ammonia in a high-pH environment. The average pH in solution is 7.5–8.[5] The typical formulation is 18-46-0 (18% N, 46% P2O5, 0% K2O).[5]
DAP can be used as a fire retardant. It lowers the combustion temperature of the material, decreases maximum weight loss rates, and causes an increase in the production of residue or char.[6] These are important effects in fighting wildfires as lowering the pyrolysis temperature and increasing the amount of char formed reduces that amount of available fuel and can lead to the formation of a firebreak.
DAP is also used as a yeast nutrient in winemaking and mead-making; as an additive in some brands of cigarettes purportedly as a nicotine enhancer; to prevent afterglow in matches, in purifying sugar; as a flux for soldering tin, copper, zinc and brass; and to control precipitation of alkali-soluble and acid-insoluble colloidal dyes on wool.[1]
Natural occurrence
The compound occurs in the nature as the exceedingly rare mineral phosphammite.[7][8] The related dihydrogen compound occurs as the mineral biphosphammite.[9][8] Both are related to guano deposits.[7][9]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.