Auwers synthesis
From Wikipedia, the free encyclopedia
The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.[1][2][3][4][5]

| Auwers synthesis | |
|---|---|
| Named after | Karl von Auwers |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000474 |
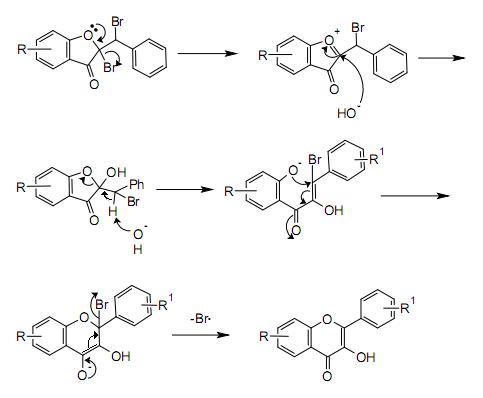
The first step in this procedure is an acid catalyzed aldol condensation between benzaldehyde and a 3-cyclooxapentanone to an o-hydroxychalcone. Bromination of the alkene group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide.
Mechanism
A possible mechanism for the rearrangement step is shown below:
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
