Tungsten oxytetrafluoride
Chemical compound From Wikipedia, the free encyclopedia
Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| WOF4 | |
| Molar mass | 275.83 g/mol |
| Appearance | colourless crystals[1] |
| Density | 5.07 g/cm3[2] |
| Melting point | 110[2] °C (230 °F; 383 K) |
| Boiling point | 185[2] °C (365 °F; 458 K) |
| reacts[2] | |
| Solubility | soluble in chloroform[3] sparingly soluble in carbon disulfide[3] |
| Structure | |
| monoclinic | |
| Related compounds | |
Other anions |
Tungsten(VI) oxytetrachloride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
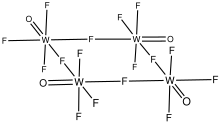
As confirmed by X-ray crystallography, WOF4 crystallizes as a tetramer. The oxides are terminal, and four of the fluorides are bridging.[4] Its structure is similar to those for niobium pentafluoride and tantalum pentafluoride. In contrast, molybdenum oxytetrafluoride adopts a polymeric structure, although again the fluorides bridge and the oxides are terminal.[5]
In the gas state, this molecule is a monomer.[6] It can form complexes with acetonitrile and other compounds.[7][8]
Preparation
Tungsten(VI) oxytetrafluoride can be synthesized by the reaction of fluorine and tungsten trioxide.[4]
It can also be obtained by treating tungsten with a mixture of oxygen and fluorine at high temperatures.[1] Partial hydrolysis of tungsten hexafluoride will also produce WOF4.[9]
- WF6 + H2O → WOF4 + 2 HF
The reaction of tungsten(VI) oxytetrachloride and hydrogen fluoride will also produce WOF4.[3]
- WOCl4 + 4HF → WOF4 + 4HCl
WOF4 can also prepared by the reaction of lead(II) fluoride and tungsten trioxide at 700 °C.[3]
- 2PbF2 + WO3 → WOF4 + 2PbO
Tungsten(VI) oxytetrafluoride hydrolyzes into tungstic acid.[1][9]
- WOF4 + 2 H2O → WO3 + 4 HF
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
