Lead(II) fluoride
Chemical compound From Wikipedia, the free encyclopedia
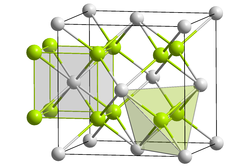
Lead(II) fluoride is the inorganic compound with the formula PbF2. It is a white solid. The compound is polymorphic, at ambient temperatures it exists in orthorhombic (PbCl2 type) form, while at high temperatures it is cubic (Fluorite type).[2]
 | |
 | |
| Names | |
|---|---|
| Other names
Lead difluoride plumbous fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.089 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| PbF2 | |
| Molar mass | 245.20 g/mol |
| Appearance | white powder |
| Odor | odorless |
| Density | 8.445 g/cm3 (orthorhombic) 7.750 g/cm3 (cubic) |
| Melting point | 824 °C (1,515 °F; 1,097 K) |
| Boiling point | 1,293 °C (2,359 °F; 1,566 K) |
| 0.057 g/100 mL (0 °C) 0.0671 g/100 mL (20 °C)[1] | |
Solubility product (Ksp) |
2.05·10−8 (20 °C) |
| Solubility | soluble in nitric acid and hydrochloric acid; insoluble in acetone and ammonia |
| −58.1·10−6 cm3/mol | |
| Structure | |
| Fluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3031 mg/kg (oral, rat) |
| Related compounds | |
Other anions |
Lead(II) chloride Lead(II) bromide Lead(II) iodide |
Other cations |
Difluorocarbene Difluorosilylene Difluorogermylene Stannous fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
Lead(II) fluoride can be prepared by treating lead(II) hydroxide or lead(II) carbonate with hydrofluoric acid:[3]
- Pb(OH)2 + 2 HF → PbF2 + 2 H2O
Alternatively, it is precipitated by adding hydrofluoric acid to a lead(II) salt solution, or by adding a fluoride salt to a lead salt, such as potassium fluoride to a lead(II) nitrate solution,[4]
- 2 KF + Pb(NO3)2 → PbF2 + 2 KNO3
or sodium fluoride to a lead(II) acetate solution.
- 2 NaF + Pb(CH3COO)2 → PbF2 + 2 NaCH3COO
Uses

2 scintillator crystals used in the Muon g−2 experiment.
Lead(II) fluoride is used in low melting glasses, in glass coatings to reflect infrared rays, in phosphors for television-tube screens, and as a catalyst for the manufacture of picoline.[3] The Muon g−2 experiment uses PbF
2 crystals in conjunction with silicon photomultipliers. High energy charged particles create Cerenkov light as they pass through the crystals, which is measured by the silicon photomultipliers.[7][8]
It also serves as a oxygen scavenger in high-temperature fluorine chemistry, as plumbous oxide is relatively volatile.[9]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
