Top Qs
Timeline
Chat
Perspective
Ferrate(VI)
Ion From Wikipedia, the free encyclopedia
Remove ads
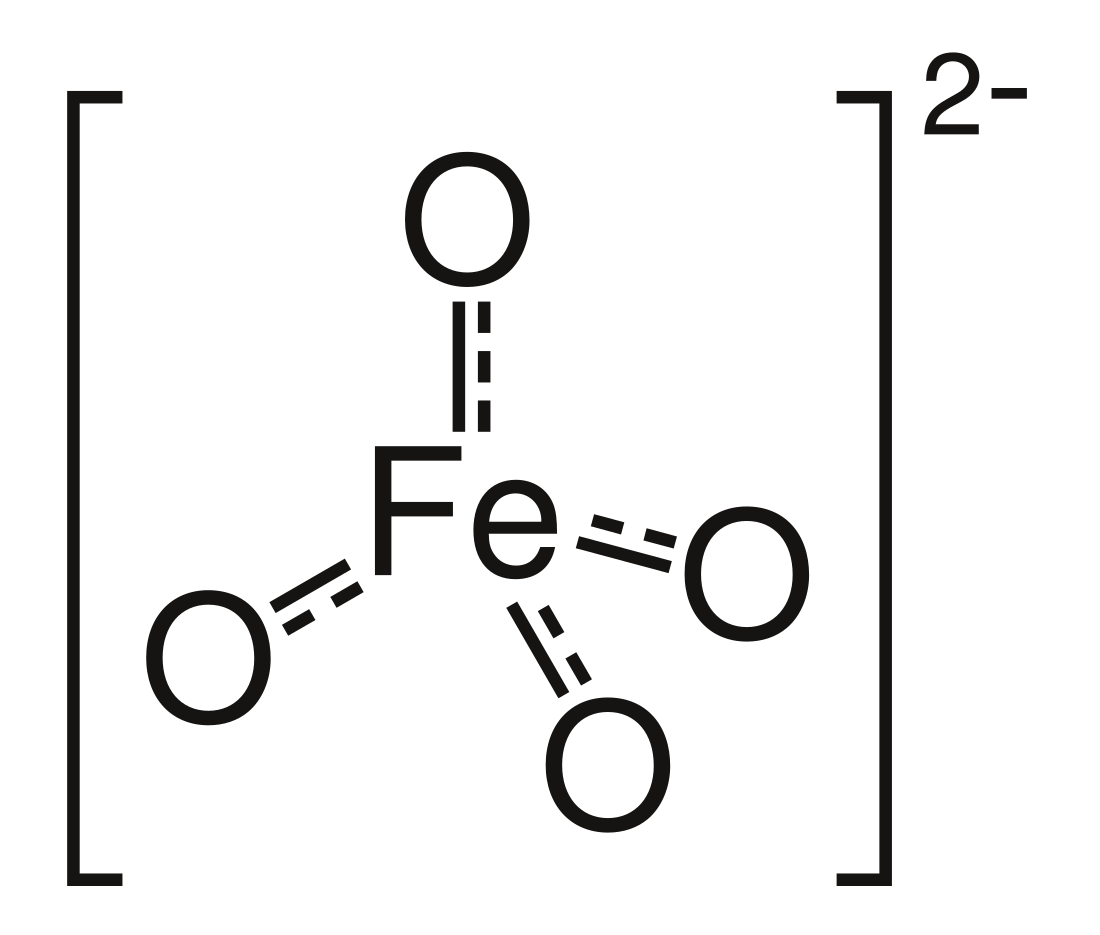
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.
Remove ads
Nomenclature
The term ferrate is normally used to mean ferrate(VI), although it can refer to other iron-containing anions, many of which are more commonly encountered than salts of [FeO4]2−.[1]
In battery electrochemistry, ferrate(VI) is sometimes called "super-iron".[2][3]
Synthesis
Ferrate(VI) salts are formed by oxidizing iron in an aqueous medium with strong oxidizing agents under alkaline conditions, or in the solid state by heating a mixture of iron filings and powdered potassium nitrate.[4]
For example, ferrates are produced by heating iron(III) hydroxide with sodium hypochlorite in alkaline solution:[5]
The anion is typically precipitated as the barium(II) salt, forming barium ferrate.[5]
Remove ads
Properties
Fe(VI) is a strong oxidizing agent over the entire pH range, with a reduction potential (Fe(VI)/Fe(III) couple) varying from +2.2 V to +0.7 V versus SHE in acidic and basic media respectively.
- [FeO
4]2−
+ 8 H+ + 3 e− ⇌ Fe3+
+ 4 H2O; E0 = +2.20 V (acidic medium) - [FeO
4]2−
+ 4 H2O + 3 e− ⇌ Fe(OH)
3 + 5 OH−
; E0 = +0.72 V (basic medium)
Because of this, the ferrate(VI) anion is unstable at neutral[4] or acidic pH values, decomposing to iron(III):[5] The reduction goes through intermediate species in which iron has oxidation states +5 and +4.[6] These anions are even more reactive than ferrate(VI).[7] In alkaline conditions ferrates are more stable, lasting for about 8 to 9 hours at pH 8 or 9.[7]
Aqueous solutions of ferrates are pink when dilute, and deep red or purple at higher concentrations.[6][8] The ferrate ion is a stronger oxidizing agent than permanganate,[9] and oxidizes ammonia to molecular nitrogen.[10]
The ferrate(VI) ion has two unpaired electrons and is thus paramagnetic. It has a tetrahedral molecular geometry, isostructural with the chromate and permanganate ions.[6]
Applications
Ferrates(VI) are potentially an environmentally-friendly water treatment chemical, as the byproduct of ferrate oxidation is the relatively benign iron(III).[11] The strong oxidativity makes them excellent disinfectants, capable of removing and destroying viruses[12] and other microbes, arsenic, sulfur-containing compounds, cyanides, nitrogen-containing contaminants, many organic compounds, and algae.[13]
Sodium ferrate (Na2FeO4) is a useful reagent with good selectivity and is stable in aqueous solution of high pH, remaining soluble in an aqueous solution saturated with sodium hydroxide.[citation needed]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads