热门问题
时间线
聊天
视角
手性助剂
来自维基百科,自由的百科全书
Remove ads
手性助劑是一種在有机合成中為了控制的合成产物立体构型而暫時引入的化合物或结构單元。[1][2]手性助劑的手性结构会影响一个或多个后续反应的立体选择性。而且引入的手性助剂通常可通过脱除来回收利用。

手性助劑於不對稱合成中的反應通式
大部分的生物分子和標靶藥物为兩種可能的鏡像異構体(又稱對映異構)中一種,因此这类天然产物和药物的合成通常以得到单一手性产物为目标,即纯度为对映纯或光学纯(enantiomerically pure,enantiopure)[3]。为了达到这一目标,采用手性助剂就是一种常用的合成方法[4]。
1975年艾里亞斯·詹姆斯·科里用手8-苯基薄荷醇(chiral 8-phenylmenthol)为例首次向人们介紹何謂手性助劑,而後1980年巴里·特羅斯特也以手性扁桃酸來介紹之。由于薄荷醇製備困難,所以在1985年J. K. Whitesell以反-2-苯基環己醇为例来介绍手性助剂。
Remove ads
非對稱合成
為了控制化合物立體中心的絕對構型,手性助劑會在合成路线中引入。在眾多使用手性助劑的合成实例中。大衛·A·伊凡斯发明的大环内酯类抗生素胞变菌素的不对称合成被認為是一个經典例子。当中采用了噁唑烷酮手性輔助劑参与了1个不对称烷基化反应和4个不对称羟醛缩合反应,得到了含有9个手性中心的绝对构型胞变菌素[5]。

大衛·A·伊凡斯於1990年合成出的胞变菌素。藍色及紅色部分表示噁唑烷酮手性助劑产生的手性中心
一個采用手性助剂进行非對稱合成的典型反應有三个步骤:第一,手性助剂与反应底物进行共价结合;其次结合产物会经历至少一个立体选择性反应;最后手性助剂在不引起产物外消旋化的情况下被移除[4]。虽然用到的对应化学计量的手性助剂成本较高,以及需额外步骤对手性助剂进行引入和脱除使得这种合成方法看起来效率很低很不划算,但对于许多化合物的合成来说,唯一可以产生立体选择性的方法必须用到手性助剂。此外,采用手性助剂的合成反应适用范围很广,且研究得较为透彻,可以最省时地获得对映体纯的产物[2]。
Remove ads
8-苯基薄荷醇
在早期使用手性助劑进行不對稱合成的範例中,艾里亞斯·詹姆斯·科里等人介绍了一种由(-)-8-苯基薄荷醇丙烯酸酯与5-苄氧甲基環戊二烯之間的不對稱狄尔斯-阿尔德环加成反应[7]。反应產物经过一系列后续反应得到一种碘代內酯化合物,这种碘代内酯是Corey法合成前列腺素的经典中间体。在反应中,因为丙烯酸酯的背面被手性助劑阻擋,所以[狄尔斯-阿尔德加成只能發生在烯烴的前方。

使用手性助劑(-)-8-苯基薄荷醇來合成前列腺素的非鏡像選擇性雙烯環加成反應。
(-)-8-苯基薄荷醇可以從任一長葉薄荷酮的对映体製備[8],即使沒有一個途徑更高效率。基于8-苯基薄荷醇在不对称合成中的廣泛使用,例如反式-2-苯基环己醇[9]和反式-2-(1-苯基-1-甲基乙基)環己醇[10]等更易合成的替代品被开发出来。
1,1'-联-2-萘酚(BINOL)
1,1'-联-2-萘酚(BINOL)自1983年起一直被用作不对称合成的手性助剂[11][12]。

山本尚首次利用(R)-BINOL作为手性助剂,对环状单萜烯进行不对称合成。以(R)-BINOL作为手性助剂,通过单硅化和烷基化反应制备出(R)-BINOL 单萜醚。随后用有机铝试剂还原,合成出产率较低(产率29%)且對映體過剩率适中(最高64% ee)的柠檬烯[12]。

京都大学化学研究所的富士薫以轴手性BINOL作为手性助剂,通过手性甘氨酸衍生物的烷基化,可以制备多种对映体纯的自然界罕见的R-氨基酸。根据不同的亲电试剂,对映体过剩率从69%到86%不等[13]。

用(R)-BINOL保护醛基,芳基乙二醛与格氏试剂进行非立体选择性反应,得到保护的阻转乳醛结构,具有中等至优异的非对映体过量和高产率[14]。

在金属催化C-P键不对称耦合得到手性磷化合物中,也用到了BINOL手性助剂。Mondal等人发现轴向手性BINOL基亚磷酰胺与芳基卤或三氟甲磺酸芳酯的Pd催化C-P交叉偶联反应中,由于BINOL存在于P反应中心附近,使得反应具有出色的立体选择性[15]。
反式-2-苯基环己醇

1985年,James K. Whitesell等人发明了一种基于反式-2-苯基环己醇结构单元的手性助剂,并在烯反应中采用了其乙醛酸酯作为手性助剂[16]。

天然产物(−)-heptemerone B和(−)-guanacastepene E的全合成中,就用到了反式-2-苯基环己醇手性助剂。乙醛酸反式-2-苯基环己醇酯与2,4-二甲基-2-戊烯在四氯化锡作用下得到以反式加成为主的产物(反式:顺式=10:1)[17]。

为了更好地控制构型,将苯基换成三苯甲基可得到反式-2-三苯甲基环己醇(TTC)。2015年,Browm研究组发表了一种高效的手性高锰酸盐介导的环氧化反应,其中就使用了TTC[18]。

噁唑烷酮
噁唑烷酮类手性助剂由大衛·A·伊凡斯推广,现如今已被應用到許多立體選擇性合成上,包括羥醛縮合反應[19]、烷基化反應,[20]和狄爾斯 - 阿爾德反應[21][22]等。这类助剂中,噁唑烷酮的4和5号位被取代基取代,并透過空間位阻引導了各種基團取代的方向。而噁唑烷酮助劑可透過水解等方式进行脱除。
噁唑烷酮类助剂可以從胺基酸或從容易取得的氨基醇中製備得到。而且量大的噁唑烷酮类助剂也均有市售,包括以下四種:
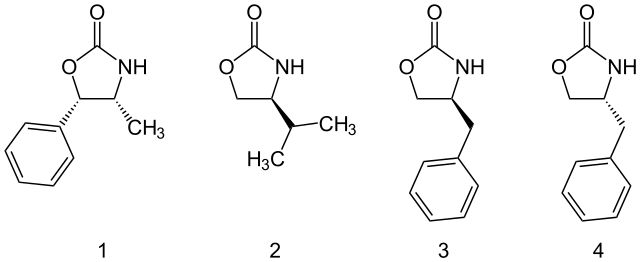
一些可購買的噁唑烷酮手性助劑。

手性噁唑烷酮采用丙酰氯进行酰化。
使用二异丙基氨基锂等强碱对噁唑烷酮酰亚胺的α-碳进行去质子化能選擇性地产生(Z)-烯醇盐,其可以进行立体选择性烷基化反应。

噁唑烷酮酰亞胺用芐基溴的烷基化反应。
不对称羥醛縮合最常用的手性助剂就是噁唑烷酮类助劑。
采用路易斯酸三氟甲磺酸二丁硼(Bu2BOTf)和二異丙基乙胺(i-Pr2NEt)作為鹼对噁唑烷酮类助劑进行软烯醇化,得到相应的(Z)-烯醇产物。而此化合物可与醛进行非对称羟醛缩合产生连个相邻的手性中心。

立體選擇性的伊凡斯羥醛縮合反應。
其反应机理可用羟醛缩合Zimmerman-Traxler过渡态模型解释,如下图所示,甲基和仲醇在Zimmerman-Traxler六元环过渡态中处于顺位(syn)关系,烯醇氧原子和醛基氧原子同时与硼原子相连。醛发生取向使得氢处于准-轴向位(pseudo-axial),以最大限度地减少1,3-二轴相互作用。两个立体中心的绝对立体构型由手性助剂分子的手性控制。在过渡态结构中,手性助剂的羰基远离烯醇氧,以使分子的净偶极最小化;烯醇的一个面被手性助剂分子上的取代基阻挡,产生立体选择性。

伊凡斯不对称羥醛缩合的Zimmerman-Traxler过渡态模型
Remove ads
噁唑烷酮助剂有多种脱除方法,進而可以產生不同的官能基。

噁唑烷酮醯亞胺通过多种脱除方式产生不同的官能基。
樟脑磺内酰胺
樟脑磺内酰胺也是一种经典的手性助剂。

大阪市立大学的大船泰史研究组在不对称全合成大环脂肽抗生素manzacidin B中,就用到了樟脑磺内酰胺作为手性助剂得到manzacidin B中核心噁唑啉结构。相比于𫫇唑烷酮助剂,樟脑磺内酰胺有更高的(2S,3R)构型选择性[23]。

樟脑磺内酰胺在不对称迈克尔加成中也有运用。在有机锂强碱的作用下,硫醇与N-甲基丙烯酰樟脑磺酰胺发生立体选择性迈克尔加成,得到较高的非立体选择性加成产物[24]。

樟脑磺内酰胺也可用在不对称克莱森重排反应中。以2,6-二叔丁基对甲酚作为自由基淬灭剂,香叶醇与樟脑磺内酰胺的甲苯溶液装入密封管中以140°C加热,以72%的产率生成主要重排产物 (2R,3S)异构体,产生包括两个相邻季碳立体中心[25]。

Remove ads
偽麻黃鹼和偽麻黃醯胺
(R,R)-和(S,S)-偽麻黃鹼皆可作為手性助劑。[26]偽麻黃鹼與羧酸、酸酐和醯氯反應形成偽麻黃醯胺(pseudoephedrine amide)。
羰基的α-氢原子很容易被非親核鹼移除产生烯醇化物。加成化合物(例如烷基卤化物)的构型由甲基决定。因此,任何加成产物都将与甲基呈顺式(syn),与羟基呈反式(anti)。之后可通过使用适当的亲核试剂裂解酰胺键来脱除伪麻黄碱手性助剂。
偽麻黃鹼的兩種对映体皆有市售。因為偽麻黃鹼可以用于制备非法物質甲基苯丙胺,所以无论是學術用途还是工業用途,购买偽麻黃鹼都收到严厉的监管。为了克服这一点,Myers等人开发利用伪麻黄酰胺作为烷基化手性助剂的替代方法[27]。即使没有市售的伪麻黄酰胺,但其可由二苯基乙二酮进行简单合成得到,而且不能用于合成甲基苯丙胺。

偽麻黃鹼和伪麻黄酰胺手性助劑。

(S,S)-偽麻黃鹼使用酸酐醯化得到对应的偽麻黃醯胺
偽麻黃醯胺藉由二異丙基胺基鋰(LDA)等強鹼進行去質子反應而得到相應的(Z)-烯醇鋰盐。采用這些烯醇鋰盐进行烷基化擁有很高的反应面選擇性。

偽麻黃醯胺的立体选择性烷基化。
偽麻黃醯胺的这种反应面選擇性常被認為是其結構所致,因為烯醇鋰的其中一個面被烷基仲醇锂以及溶剂化锂离子阻挡,烷基化反应倾向于在另一面进行。根据这一理论,产物立体选择性与氯化锂和溶剂四氢呋喃用量高度相关。通常,4至6摩尔当量的氯化锂足以在反应浓度下使THF中的烯醇盐溶液饱和。

偽麻黃醯胺烯醇的面选择性烷基化图例。
伪麻黄碱酰胺不对称烷基化的主要优势之一是酰胺烯醇盐通常具有足够的亲核性,可在 -78 °C 至 0 °C 的温度下与伯卤甚至叔卤化物发生反应。通过α-支链酰胺烯醇盐的烷基化构建季碳手性中心也是可能的,但对于反应性较低的亲电试剂,必须添加DMPU[29]。
目前已經發展出將偽麻黃醯胺轉化成不同类型对映体化合物的脱除方法,如羧酸、醇、醛和酮類。

將偽麻黃醯胺通过不同脱除方法产生不同类型化合物。
反應完成後,被脱除的偽麻黃醯胺可以重新利用。
叔丁基亞磺醯胺
乔纳森·埃尔曼(Jonathan Ellman)研究组已經广泛研究出利用手性亞磺醯胺(sulfonamide)衍生物作為手性助劑的方法。[30]

叔丁基亞磺醯胺的兩個对映異構体。
任一叔丁基亞磺醯胺的对映異構体可藉由叔丁基二硫经两步合成得到:催化不对称氧化反应以高产率和对映体选择性生成二硫键氧化产物硫代亚磺酸酯。接著以在氨基锂的氨溶液中處鋰該化合物,即可得到光学纯的逆产物。

由常见易得的叔丁基二硫合成的叔丁基亞磺醯胺方法。
以醛或酮來進行叔丁基亞磺醯胺的縮合反應,會以高產率和高对映体过剩率地得到(E)-異構体的N-亞磺醯醛亚胺或N-亞磺醯酮亚胺。

叔丁基亞磺醯胺与醛或酮的縮合得到对应的N-亞磺醯亚胺。
将格氏试剂加成到叔丁烷亚磺酰基醛亚胺或酮亚胺中,可发生不对称加成生成支链亚磺酰胺。该现象可以用六元环过渡态模型进行合理解释,其中亞磺醯亞胺的氧與氮皆會與鎂配位。

叔丁基亞磺醯胺与格氏試劑的加成反应。

亞磺醯胺輔助劑的酸解。
SAMP与RAMP
手性体(S)-1-氨基-2-(甲氧基甲基)吡咯(SAMP)和(R)-1-氨基-2-(甲氧基甲基)吡咯(RAMP)的腙烷基化反應,是由迪特·恩德斯和艾里亞斯·詹姆斯·科里研發。[31][32]
SAMP可藉由(S)-脯胺酸经六步反应製備,而RAMP則可藉由(R)-谷胺酸经六步反应製備。

用市售试剂製備SAMP與RAMP。
SAMP或RAMP经与醛或酮縮合反應會得到相应(E)-肼类化合物,随后利用强碱LDA去質子化以及鹵代烷加成就得到相应烷基化产物。手性助剂可由臭氧化反應或水解进行脱除。

SAMP或RAMP手性助劑的縮合、烷基化與脱除。
工業中的手性助劑
手性助剂通常可靠且用途广泛,能够以省时的方式合成大量对映体纯化合物。因此手性助剂通常是药物开发早期阶段的首选方法[2]。
HIV蛋白酶抑制劑替拉那韦是一种已上市的治疗HIV感染药物。替拉那韦的首个立体选择性合成路线涉及有机铜酸盐与手性迈克尔受体进行迈克尔共轭加成[33]。迈克尔受体中的手性恶唑烷酮结构控制替拉那韦分子中两个手性中心当中一个的立体构型。然而替拉那韦的最终商业化合成路线没有用到手性助剂;这个手性中心反而是利用不对称加氢反应得到的[34]。

替拉那韦形成關鍵立體中心的最初以及最终合成策略。
阿托伐他汀的鈣鹽在市場上的商品名為立普妥(Lipitor),是一種降血中膽固醇的藥物。阿托伐他汀的首个立体选择性药物合成路线依赖于手性酯的非立体选择性醇醛缩合反应来控制两个醇立体中心其中一个立体构型[35]。在阿托伐他汀的商业合成路线中,这一手性中心构型则是从常用的食品添加剂異抗壞血酸的手性继承得到[36]。

阿托伐他汀的關鍵立體中心的最初以及商业合成策略。
另請參見
- 反式-2-苯基-1-環己醇作為手性助劑的合成範例:尾島內酯
- 纈胺酸在Schöllkopf方法中擔任手性助劑。
參考
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads