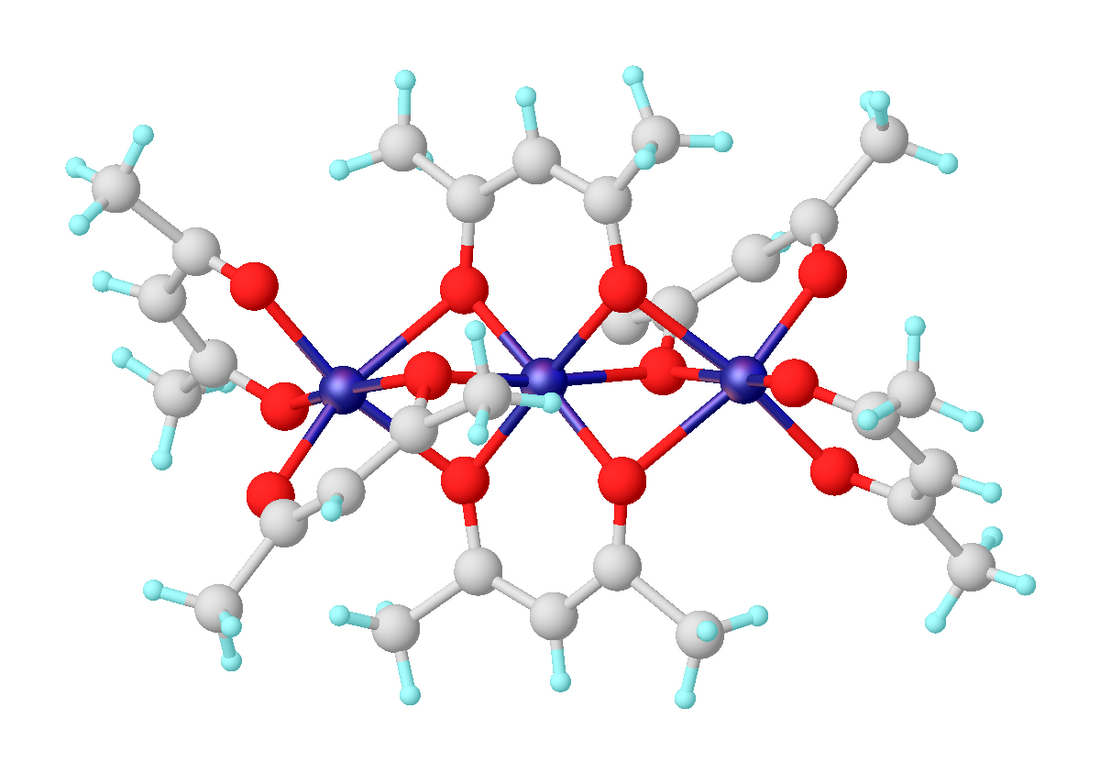
乙醯丙酮鎳是一種配位化合物,化學式為[Ni(acac)2]3,其中acac表示乙醯丙酮陰離子C5H7O2−。它是暗綠色的順磁性固體,可溶於有機溶劑(如甲苯)。它和水反應,生成藍綠色的二水合物Ni(acac)2(H2O)2。[1]

製備
乙醯丙酮鎳的二水合物可由硝酸鎳和乙醯丙酮在鹼的存在下反應得到:[2]
- Ni(NO3)2 + 2 CH3COCH2COCH3 + 2 H2O + 2 NaOH → Ni(CH3COCHCOCH3)2(H2O)2 + 2 NaNO3
這個配合物可以通過迪安-斯塔克裝置進行共沸蒸餾來脫水:[2]
- 3 Ni(CH3COCHCOCH3)2(H2O)2 → [Ni(CH3COCHCOCH3)2]3 + 6 H2O
Ni(acac)2(H2O)2在170–210 °C和0.2~0.4 mmHg的壓力下升華,同時生成無水物。[3]
參考文獻
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.