Methane sī siōng kan-tan-ê chi̍t-khóaⁿ Alkane, i-ê hòa-ha̍k-sek siá-chòe CH4. 1-ê methane sī 1-ê carbon keng-iû 4-ê sp3 hūn-sêng kúi-tō lâi hām 4-ê hydrogen só͘ chó͘-sêng ê hoà-ha̍p-bu̍t. Methane sī thian-jiân-khì ê chú-iàu sêng-hun. In-ūi methane ū siong-tùi kah koâiⁿ-ê sán-liōng, só͘-í i sī chi̍t-ê chin jia̍t-mn̂g-ê jiân-liāu; put-kò, siu-chi̍p methane ē khì tú-tio̍h kī-su̍t-ê thiau-chiàn, in-ūi methane tī phiau-chún chōng-hóng chi-hā sī khì-thé.
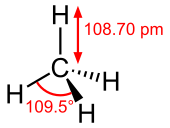 | |||
| |||
| Hō-miâ | |||
|---|---|---|---|
| Iu-sian ê IUPAC hō-miâ
Methane[1] | |||
| Hē-thóng-tek IUPAC hō-miâ
Carbane (never recommended[1]) | |||
Kî-tha hō-miâ
| |||
| Sek-pia̍t-hō | |||
CAS Number |
|||
3D model (JSmol) |
|||
| 3DMet | B01453 | ||
Beilstein Reference |
1718732 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.739 | ||
| EC Number | 200-812-7 | ||
Gmelin Reference |
59 | ||
| KEGG | |||
| MeSH | Methane | ||
PubChem CID |
|||
| RTECS number | PA1490000 | ||
| UNII | |||
| UN number | 1971 | ||
InChI
| |||
SMILES
| |||
| Sèng-chit | |||
| CH4 | |||
| Mole chit-liōng | 16.04 g·mol−1 | ||
| Gōa-māu | Colorless gas | ||
| Khì-bī | Odorless | ||
| Bi̍t-tō͘ | |||
| Iûⁿ-tiám | −182.456 °C (−296.421 °F; 90.694 K)[3] | ||
| Hut-tiám | −161.5 °C (−258.7 °F; 111.6 K)[3] | ||
Tī chúi ê iûⁿ-kái-tō͘ |
22.7 mg·L−1[4] | ||
| Iûⁿ-kái-tō͘ | Soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone and insoluble in water | ||
| log P | 1.09 | ||
Henry's law constant (kH) |
14 nmol·Pa−1·kg−1 | ||
| Conjugate acid | Methanium | ||
| Conjugate base | Methyl anion | ||
Chû-hòa-lu̍t (χ) |
−17.4×10−6 cm3·mol−1[5] | ||
| Kò͘-chō | |||
Point group |
Td | ||
Molecular shape |
Tetrahedron | ||
Dipole moment |
0 D | ||
| Jia̍t-hòa-ha̍k[6] | |||
Heat capacity (C) |
35.7 J·(K·mol)−1 | ||
Piau-chún mole entropy (S |
186.3 J·(K·mol)−1 | ||
Piau-chún hêng-sêng enthalpy (ΔfH |
−74.6 kJ·mol−1 | ||
Std enthalpy of combustion (ΔcH |
−891 kJ·mol−1 | ||
| Gûi-hiám[7] | |||
| GHS pictograms |  | ||
| GHS signal word | DANGER | ||
GHS hazard statements |
H220 | ||
GHS precautionary statements |
P210 | ||
| NFPA 704 | 
4
2
0 SA | ||
| Ín-hóe-tiám | −188 °C (−306.4 °F; 85.1 K) | ||
Autoignition temperature |
537 °C (999 °F; 810 K) | ||
| Explosive limits | 4.4–17% | ||
| Koan-liân hòa-ha̍p-bu̍t | |||
Related alkanes |
| ||
Tû-liáu te̍k-pia̍t chí chhut, chu-liāu sī kun-kù bu̍t-chit ê piau-chún chōng-thài (tī 25 °C [77 °F], 100 kPa). | |||
| Infobox chham-chiàu | |||
Tsù-kái
Tsham-khó bûn-hèn
Guā-pōo lên-ket
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.



