IDRA-21是种AMPA受体的正别构调节剂,也是苯丙噻二嗪的衍生物,也是一种手性分子,(+)-IDRA-21是其活性形式。[1]
Quick Facts 法律规范状态, 法律规范 ...
IDRA-21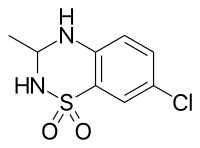 |
|
| 法律规范 |
|
|---|
|
7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide
|
| CAS号 | 22503-72-6  Y Y |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| 化学式 | C8H9ClN2O2S |
|---|
| 摩尔质量 | 232.68 g·mol−1 |
|---|
| 3D模型(JSmol) | |
|---|
CC1NC2=C(C=C(C=C2)Cl)S(=O)(=O)N1
|
InChI=1S/C8H9ClN2O2S/c1-5-10-7-3-2-6(9)4-8(7)14(12,13)11-5/h2-5,10-11H,1H3  N NKey:VZRNTCHTJRLTMU-UHFFFAOYSA-N  N N
|
Close
在动物实验中,IDRA-21表现出了有促进学习的效果,并能明显改善学习和记忆。在逆转阿普唑仑或东莨菪碱诱发的认知障碍方面,有着约比茴拉西坦10到30倍的效力,[2][3]且在单次服用后,可产生长达48小时的持续效应,[4]其作用机制,被认为是通过促进大脑突触间的诱导LTP达到的。[5]
正常情况下,IDRA-21或许不产生神经毒性,[6]但可能会加重中风或癫痫发作后,因全身缺血所造成的神经元损伤。[7]
与安帕金或苯甲酰哌啶衍生的AMPA受体增效剂相比,IDRA-21药效优于CX-516,低于CX-546。[8]与IDRA-21相比,效力更好的苯丙噻二嗪类衍生物已被开发出来,[9][10]但这些衍生物,各所获的研究程度并不一样,苯甲酰基哌啶(benzoylpiperidine)和苯甲酰基吡咯烷CX系列(benzoylpyrrolidine CX-series)药物,在临床开发中更受青睐,这或是由于它们在高剂量使用时,具有更有利的毒性特征。[11]
Uzunov DP, Zivkovich I, Pirkle WH, Costa E, Guidotti A. Enantiomeric resolution with a new chiral stationary phase of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide, a cognition-enhancing benzothiadiazine derivative. Journal of Pharmaceutical Sciences. August 1995, 84 (8): 937–42. PMID 7500277. doi:10.1002/jps.2600840807. Thompson DM, Guidotti A, DiBella M, Costa E. 7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proceedings of the National Academy of Sciences of the United States of America. August 1995, 92 (17): 7667–71. Bibcode:1995PNAS...92.7667T. PMC 41206  . PMID 7644474. doi:10.1073/pnas.92.17.7667
. PMID 7644474. doi:10.1073/pnas.92.17.7667  .
. Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A. 7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. The Journal of Pharmacology and Experimental Therapeutics. January 1995, 272 (1): 300–9. PMID 7815345. Arai AC, Xia YF, Kessler M, Phillips D, Chamberlin R, Granger R, Lynch G. Effects of 5'-alkyl-benzothiadiazides on (R,S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor biophysics and synaptic responses. Molecular Pharmacology. September 2002, 62 (3): 566–77. PMID 12181433. S2CID 16182942. doi:10.1124/mol.62.3.566.
