Sulfurous acid
chemical compound From Wikipedia, the free encyclopedia
Sulfurous acid is a weak acid with the chemical formula H2SO3. It is produced by dissolving sulfur dioxide in water. Bases deprotonate (remove the Hydrogen ion) it to produce sulfites. It tends to turn back into sulfur dioxide and water again. It is a weak reducing agent.
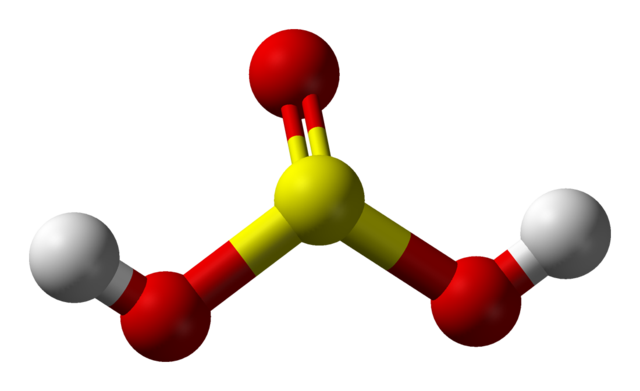
Sulfurous acid has two tautomers, or chemical structures that easily change into each other. One is the SO(OH)2 form, and the other is a sulfonic acid, HSO2OH. The bisulfite ion, when sulfurous acid loses only one hydrogen atom, has the shape of the sulfonic acid tautomer.[1]
Related pages
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
