Naphthalene
chemical compound From Wikipedia, the free encyclopedia
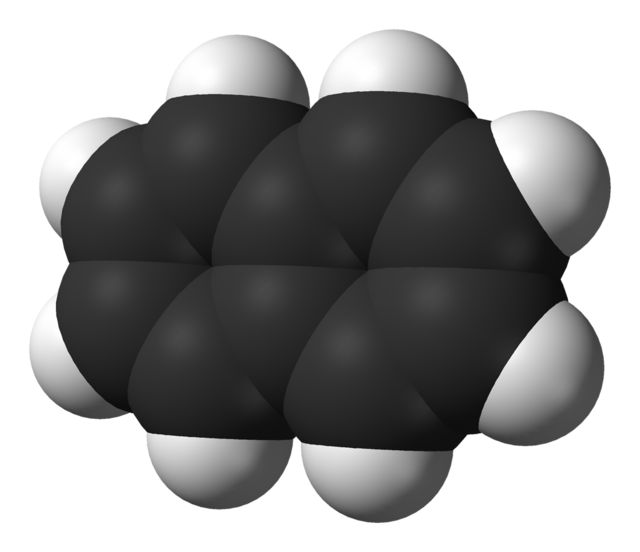
Naphthalene is a crystalline, white hydrocarbon, with a strong smell. It is best known as the main ingredient in mothballs, urinal deodorizer blocks, and can be used as an antiseptic. In mothballs, it is used as an insecticide or pesticide.
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Naphthalene[1] | |||
| Systematic IUPAC name
Bicyclo[4.4.0]deca-1,3,5,7,9-pentaene | |||
| Other names
white tar, camphor tar, tar camphor, naphthalin, naphthaline, antimite, albocarbon, hexalene, mothballs, moth flakes | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| Beilstein Reference | 1421310 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.863 | ||
| EC Number |
| ||
| Gmelin Reference | 3347 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
SMILES
| |||
| Properties | |||
| C10H8 | |||
| Molar mass | 128.17 g·mol−1 | ||
| Appearance | White solid crystals/ flakes | ||
| Odor | Strong odor of coal tar | ||
| Density | 1.145 g/cm3 (15.5 °C)[2] 1.0253 g/cm3 (20 °C) 0.9625 g/cm3 (100 °C)[2] | ||
| Melting point | 78.2 °C (172.8 °F; 351.3 K) 80.26 °C (176.47 °F; 353.41 K) at 760 mmHg | ||
| Boiling point | 217.97 °C (424.35 °F; 491.12 K) at 760 mmHg[2] | ||
| 19 mg/L (10 °C) 31.6 mg/L (25 °C) 43.9 mg/L (34.5 °C) 80.9 mg/L (50 °C)> 238.1 mg/L (73.4 °C)[3] | |||
| Solubility | Soluble in alcohols, liquid ammonia, carboxylic acids, C6H6, SO2,[3] CCl4, CS2, toluene, aniline[4] | ||
| Solubility in ethanol | 5 g/100 g (0 °C) 11.3 g/100 g (25 °C) 19.5 g/100 g (40 °C) 179 g/100 g (70 °C)[4] | ||
| Solubility in acetic acid | 6.8 g/100 g (6.75 °C) 13.1 g/100 g (21.5 °C) 31.1 g/100 g (42.5 °C) 111 g/100 g (60 °C)[4] | ||
| Solubility in chloroform | 19.5 g/100 g (0 °C) 35.5 g/100 g (25 °C) 49.5 g/100 g (40 °C) 87.2 g/100 g (70 °C)[4] | ||
| Solubility in hexane | 5.5 g/100 g (0 °C) 17.5 g/100 g (25 °C) 30.8 g/100 g (40 °C) 78.8 g/100 g (70 °C)[4] | ||
| Solubility in butyric acid | 13.6 g/100 g (6.75 °C) 22.1 g/100 g (21.5 °C) 131.6 g/100 g (60 °C)[4] | ||
| log P | 3.34 | ||
| Vapor pressure | 8.64 Pa (20 °C) 23.6 Pa (30 °C) 0.93 kPa (80 °C)[3] 2.5 kPa (100 °C)[5] | ||
| kH | 0.42438 L·atm/mol | ||
| -91.9·10−6 cm3/mol | |||
| Thermal conductivity | 98 kPa: 0.1219 W/m·K (372.22 K) 0.1174 W/m·K (400.22 K) 0.1152 W/m·K (418.37 K) 0.1052 W/m·K (479.72 K)[6] | ||
Refractive index (nD) |
1.5898 | ||
| Viscosity | 0.964 cP (80 °C) 0.761 cP (100 °C) 0.217 cP (150 °C)[7] | ||
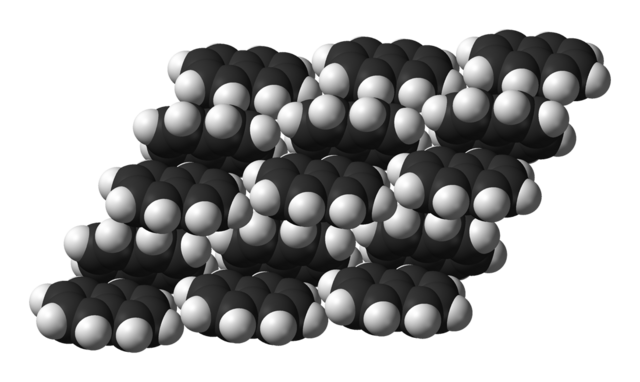
| Structure | |||
| Monoclinic[8] | |||
Space group |
P21/b[8] | ||
| C5 2h[8] | |||
Lattice constant |
α = 90°, β = 122.92°, γ = 90°
| ||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
78.53 kJ/mol | ||
| Std enthalpy of combustion ΔcH |
-5156.3 kJ/mol | ||
| Standard molar entropy S |
167.39 J/mol·K[5] | ||
| Specific heat capacity, C | 165.72 J/mol·K | ||
| Hazards | |||
| Main hazards | Flammable, sensitizer, possible carcinogen. Dust can form explosive mixtures with air | ||
| NFPA 704 |
| ||
| Explosive limits | 5.9% | ||
| U.S. Permissible exposure limit (PEL) |
TWA 10 ppm (50 mg/m3) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| verify (what is ?) | |||
| Infobox references | |||
Naphthalene is toxic. In humans, being exposed to naphthalene can destroy red blood cells. Naphthalene may also cause cancer.
History
In the early 1820s, two different papers were published on something that matched the description of Naphthalene. Both groups made it by distilling coal tar. In 1821 John Kidd cited both reports, and condensed their results to accurately describe the properties of naphthalene, and how to make it. Kidd named it naphthalene because "naphtha" means any explosive hydrocarbon mixture. By 1826, Michael Faraday discovered the formula for it. Emil Erlenmeyer proposes that it is two fused benzene rings in 1866, and Carl Gräbe confirms this three years later.
References
Other websites
Wikiwand - on
Seamless Wikipedia browsing. On steroids.