Formic acid
chemical compound From Wikipedia, the free encyclopedia
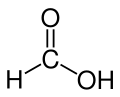
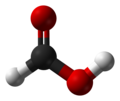
Formic acid, or methanoic acid, is the simplist carboxylic acid and has the chemical formula HCO
2H. Many animals use for defence. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies, and the trivial name in some languages means "ant-acid", such as Dutch mierenzuur, Danish myresyre, Faroese meyrusýra, Français acide formique and German Ameisensäure. Esters, salts, and the anions derived from formic acid are called formates.
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Formic acid[1] | |||
| Systematic IUPAC name
Methanoic acid[1] | |||
| Other names
Carbonous acid; Formylic acid; Hydrogen carboxylic acid; Hydroxy(oxo)methane; Metacarbonoic acid; Oxocarbinic acid; Oxomethanol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.527 | ||
| EC Number |
| ||
| E number | E236 (preservatives) | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
SMILES
| |||
| Properties | |||
| CH2O2 | |||
| Molar mass | 46.03 g·mol−1 | ||
| Appearance | Colorless fuming liquid | ||
| Odor | Pungent, penetrating | ||
| Density | 1.220 g/mL | ||
| Melting point | 8.4 °C (47.1 °F; 281.5 K) | ||
| Boiling point | 100.8 °C (213.4 °F; 373.9 K) | ||
| Miscible | |||
| Solubility | Miscible with ether, acetone, ethyl acetate, glycerol, methanol, ethanol Partially soluble in benzene, toluene, xylenes | ||
| log P | −0.54 | ||
| Vapor pressure | 35 mmHg (20 °C)[2] | ||
| Acidity (pKa) | 3.77[3] | ||
| Conjugate base | Formate | ||
| -19.90·10−6 cm3/mol | |||
Refractive index (nD) |
1.3714 (20 °C) | ||
| Viscosity | 1.57 cP at 268 °C | ||
| Structure | |||
| Planar | |||
| 1.41 D (gas) | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−425.0 kJ/mol | ||
| Std enthalpy of combustion ΔcH |
−254.6 kJ/mol | ||
| Standard molar entropy S |
131.8 J/mol K | ||
| Pharmacology | |||
ATCvet code |
QP53AG01 (WHO) | ||
| Hazards | |||
| Main hazards | Corrosive; irritant; sensitizer | ||
| NFPA 704 |
| ||
| R-phrases | Template:R10 R35 | ||
| S-phrases | (S1/2) S23 S26 S45 | ||
| Explosive limits | 14–34%[source?] 18%–57% (90% solution)[2] | ||
| U.S. Permissible exposure limit (PEL) |
TWA 5 ppm (9 mg/m3)[2] | ||
| Related compounds | |||
| Related {{{label}}} | {{{value}}} | ||
| Related compounds | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| verify (what is ?) | |||
| Infobox references | |||

In the 15th century, many alchemists reported that ants use an acidid fluid for defense. English naturalist John Ray was the first to get formic acid, by distilling ants, in 1671.
In nature, it is found in most ants.[4] The wood ants from the genus Formica can spray formic acid on their preys or to defend the nest. It is also known from the trichomes of stinging nettle (Urtica dioica). Formic acid is a naturally occurring component of the atmosphere due primarily to forest emissions.[5]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.