Loading AI tools
Allergy medication From Wikipedia, the free encyclopedia
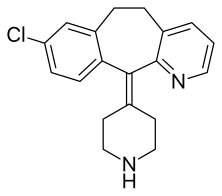
Desloratadine. sold under the brand name Clarinex among others, is a tricyclic H1 inverse agonist that is used to treat allergies. It is an active metabolite of loratadine.[6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Clarinex, Aerius, Allex, others[1] |
| Other names | descarboethoxyloratadine[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Rapidly absorbed |
| Protein binding | 83 to 87% |
| Metabolism | UGT2B10, CYP2C8 |
| Metabolites | 3-Hydroxydesloratadine |
| Onset of action | within 1 hour[6] |
| Elimination half-life | 27 hours,[6] 33.7 hours in elderly patients[3] |
| Duration of action | up to 24 hours[6] |
| Excretion | 40% as conjugated metabolites into urine Similar amount into the feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.554 |
| Chemical and physical data | |
| Formula | C19H19ClN2 |
| Molar mass | 310.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was patented in 1984 and came into medical use in 2001.[7] It was brought to the market in the US by Schering Corporation, later named Schering-Plough.[3]
Desloratadine is used to treat allergic rhinitis, nasal congestion and chronic idiopathic urticaria (hives).[8] It is the major metabolite of loratadine and the two drugs are similar in safety and effectiveness.[8] Desloratadine is available in many dosage forms and under many brand names worldwide.[9]
An emerging indication for desloratadine is in the treatment of acne, as an inexpensive adjuvant to isotretinoin and possibly as maintenance therapy or monotherapy.[10][11]
The most common side-effects are fatigue (1.2%[12]), dry mouth (3%[12]), and headache (0.6%[12]).[8]
Co-administration with erythromycin, ketoconazole, azithromycin, fluoxetine, or cimetidine resulted in elevated blood plasma concentrations of desloratadine and its metabolite 3-hydroxydesloratadine in studies. However, no clinically relevant changes were observed.[3][13]
Desloratadine is a selective H1-antihistamine which functions as an inverse agonist at the histamine H1 receptor.[14]
At very high doses, is also an antagonist at various subtypes of the muscarinic acetylcholine receptors. This effect is not relevant for the drug's action at therapeutic doses.[15]
Desloratadine is well absorbed from the gut and reaches highest blood plasma concentrations after about three hours. In the bloodstream, 83 to 87% of the substance are bound to plasma proteins.[13]
Desloratadine is metabolized to 3-hydroxydesloratadine in a three-step sequence in normal metabolizers. First, N-glucuronidation of desloratadine by UGT2B10; then, 3-hydroxylation of desloratadine N-glucuronide by CYP2C8; and finally, a non-enzymatic deconjugation of 3-hydroxydesloratadine N-glucuronide.[16][17] Both desloratadine and 3-hydroxydesloratadine are eliminated via urine and feces with a half-life of 27 hours in normal metabolizers.[13][18]

It exhibits only peripheral activity since it does not readily cross the blood–brain barrier; hence, it does not normally cause drowsiness because it does not readily enter the central nervous system.[19]
Desloratadine does not have a strong effect on a number of tested enzymes in the cytochrome P450 system. It was found to weakly inhibit CYP2B6, CYP2D6, and CYP3A4/CYP3A5, and not to inhibit CYP1A2, CYP2C8, CYP2C9, or CYP2C19. Desloratadine was found to be a potent and relatively selective inhibitor of UGT2B10, a weak to moderate inhibitor of UGT2B17, UGT1A10, and UGT2B4, and not to inhibit UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7, UGT2B15, UGT1A7, and UGT1A8.[17]
2% of Caucasian people and 18% of people from African descent are desloratadine poor metabolizers. In these people, the drug reaches threefold higher plasma concentrations at seven hours after intake, and it has a half-life of 89 hours (compared to a 27-hour half-life in normal metabolizers). Adverse effects were reported at similar rates in poor metabolizers, suggesting that it is not clinically relevant.[13][18]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.