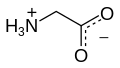
Glycine (symbol Gly or G;[6] /ˈɡlaɪsiːn/ )[7] is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable). Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG).[8] Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group. Glycine is also an inhibitory neurotransmitter[9] – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.[10]
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glycine | |||
| Systematic IUPAC name
Aminoacetic acid[2] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | Gly, G | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.248 | ||
| EC Number |
| ||
| E number | E640 (flavour enhancer) | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.067 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.1607 g/cm3[3] | ||
| Melting point | 233 °C (451 °F; 506 K) (decomposition) | ||
| 249.9 g/L (25 °C)[4] | |||
| Solubility | soluble in pyridine sparingly soluble in ethanol insoluble in ether | ||
| Acidity (pKa) | 2.34 (carboxyl), 9.6 (amino)[5] | ||
| -40.3·10−6 cm3/mol | |||
| Pharmacology | |||
| B05CX03 (WHO) | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
2600 mg/kg (mouse, oral) | ||
| Supplementary data page | |||
| Glycine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
It is the only achiral proteinogenic amino acid.[11] It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom.[12]
History and etymology
Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid.[13] He originally called it "sugar of gelatin",[14][15] but French chemist Jean-Baptiste Boussingault showed in 1838 that it contained nitrogen.[16] In 1847 American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig, proposed the name "glycocoll";[17][18] however, the Swedish chemist Berzelius suggested the simpler current name a year later.[19][20] The name comes from the Greek word γλυκύς "sweet tasting"[21] (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose). In 1858, the French chemist Auguste Cahours determined that glycine was an amine of acetic acid.[22]
Production
Although glycine can be isolated from hydrolyzed proteins, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis.[23] The two main processes are amination of chloroacetic acid with ammonia, giving glycine and hydrochloric acid,[24] and the Strecker amino acid synthesis,[25] which is the main synthetic method in the United States and Japan.[26] About 15 thousand tonnes are produced annually in this way.[27]
Glycine is also co-generated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia co-product.[28]
Chemical reactions
Its acid–base properties are most important. In aqueous solution, glycine is amphoteric: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about pH 9.6, it converts to glycinate.
Glycine functions as a bidentate ligand for many metal ions, forming amino acid complexes.[29] A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which exists both in cis and trans isomers.[30][31]
With acid chlorides, glycine converts to the amidocarboxylic acid, such as hippuric acid[32] and acetylglycine.[33] With nitrous acid, one obtains glycolic acid (van Slyke determination). With methyl iodide, the amine becomes quaternized to give trimethylglycine, a natural product:
- H
3N+
CH
2COO−
+ 3 CH3I → (CH
3)
3N+
CH
2COO−
+ 3 HI
Glycine condenses with itself to give peptides, beginning with the formation of glycylglycine:[34]
- 2 H
3N+
CH
2COO−
→ H
3N+
CH
2CONHCH
2COO−
+ H2O
Pyrolysis of glycine or glycylglycine gives 2,5-diketopiperazine, the cyclic diamide.[35]
Glycine forms esters with alcohols. They are often isolated as their hydrochloride, such as glycine methyl ester hydrochloride. Otherwise, the free ester tends to convert to diketopiperazine.
As a bifunctional molecule, glycine reacts with many reagents. These can be classified into N-centered and carboxylate-center reactions.
Metabolism
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate. In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[36]
- serine + tetrahydrofolate → glycine + N5,N10-methylene tetrahydrofolate + H2O
In E. coli, glycine is sensitive to antibiotics that target folate.[37]
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:[36]
In addition to being synthesized from serine, glycine can also be derived from threonine, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.[38]
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above. In this context, the enzyme system involved is usually called the glycine cleavage system:[36]
- Glycine + tetrahydrofolate + NAD+ ⇌ CO2 + NH+
4 + N5,N10-methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.[36]
In the third pathway of its degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.[36]
The half-life of glycine and its elimination from the body varies significantly based on dose.[39] In one study, the half-life varied between 0.5 and 4.0 hours.[39]
Physiological function
The principal function of glycine is it acts as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction with hydroxyproline.[36][40] In the genetic code, glycine is coded by all codons starting with GG, namely GGU, GGC, GGA and GGG.[8]
As a biosynthetic intermediate
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.[36]
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory.[41] The LD50 of glycine is 7930 mg/kg in rats (oral),[42] and it usually causes death by hyperexcitability.
As a toxin conjugation agent
Glycine conjugation pathway has not been fully investigated.[43] Glycine is thought to be a hepatic detoxifier of a number endogenous and xenobiotic organic acids.[44] Bile acids are normally conjugated to glycine in order to increase their solubility in water.[45]
The human body rapidly clears sodium benzoate by combining it with glycine to form hippuric acid which is then excreted.[46] The metabolic pathway for this begins with the conversion of benzoate by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[47] which is then metabolized by glycine N-acyltransferase into hippuric acid.[48]
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia ("USP"), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which may or may not meet USP grade standards, is sold at a lower price for use in industrial applications, e.g., as an agent in metal complexing and finishing.[49]
Animal and human foods

Glycine is not widely used in foods for its nutritional value, except in infusions. Instead, glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.[27]
As of 1971[update], the U.S. Food and Drug Administration "no longer regards glycine and its salts as generally recognized as safe for use in human food",[51] and only permits food uses of glycine in certain conditions.[52]
Glycine has been researched for its potential to extend life.[53][54] The proposed mechanisms of this effect are its ability to clear methionine from the body, and activating autophagy.[53]
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicides glyphosate,[55] iprodione, glyphosine, imiprothrin, and eglinazine.[27] It is used as an intermediate of antibiotics such as thiamphenicol.[citation needed]
Laboratory research
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis.[56] Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required.[57] This process is known as stripping.
Presence in space
The presence of glycine outside the Earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft Stardust from comet Wild 2 and subsequently returned to Earth. Glycine had previously been identified in the Murchison meteorite in 1970.[58] The discovery of glycine in outer space bolstered the hypothesis of so-called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe.[59] In 2016, detection of glycine within Comet 67P/Churyumov–Gerasimenko by the Rosetta spacecraft was announced.[60]
The detection of glycine outside the Solar System in the interstellar medium has been debated.[61]
Evolution
Glycine is proposed to be defined by early genetic codes.[62][63][64][65] For example, low complexity regions (in proteins), that may resemble the proto-peptides of the early genetic code are highly enriched in glycine.[65]
Presence in foods
| Food | Percentage content by weight (g/100g) |
|---|---|
| Snacks, pork skins | 11.04 |
| Sesame seeds flour (low fat) | 3.43 |
| Beverages, protein powder (soy-based) | 2.37 |
| Seeds, safflower seed meal, partially defatted | 2.22 |
| Meat, bison, beef and others (various parts) | 1.5–2.0 |
| Gelatin desserts | 1.96 |
| Seeds, pumpkin and squash seed kernels | 1.82 |
| Turkey, all classes, back, meat and skin | 1.79 |
| Chicken, broilers or fryers, meat and skin | 1.74 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 1.71 |
| Bacon and beef sticks | 1.64 |
| Peanuts | 1.63 |
| Crustaceans, spiny lobster | 1.59 |
| Spices, mustard seed, ground | 1.59 |
| Salami | 1.55 |
| Nuts, butternuts, dried | 1.51 |
| Fish, salmon, pink, canned, drained solids | 1.42 |
| Almonds | 1.42 |
| Fish, mackerel | 0.93 |
| Cereals ready-to-eat, granola, homemade | 0.81 |
| Leeks, (bulb and lower-leaf portion), freeze-dried | 0.7 |
| Cheese, parmesan (and others), grated | 0.56 |
| Soybeans, green, cooked, boiled, drained, without salt | 0.51 |
| Bread, protein (includes gluten) | 0.47 |
| Egg, whole, cooked, fried | 0.47 |
| Beans, white, mature seeds, cooked, boiled, with salt | 0.38 |
| Lentils, mature seeds, cooked, boiled, with salt | 0.37 |
See also
References
Further reading
External links
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.