Top Qs
Timeline
Chat
Perspective
Zirconium tetrafluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride is a component of ZBLAN fluoride glass.[2]
Remove ads
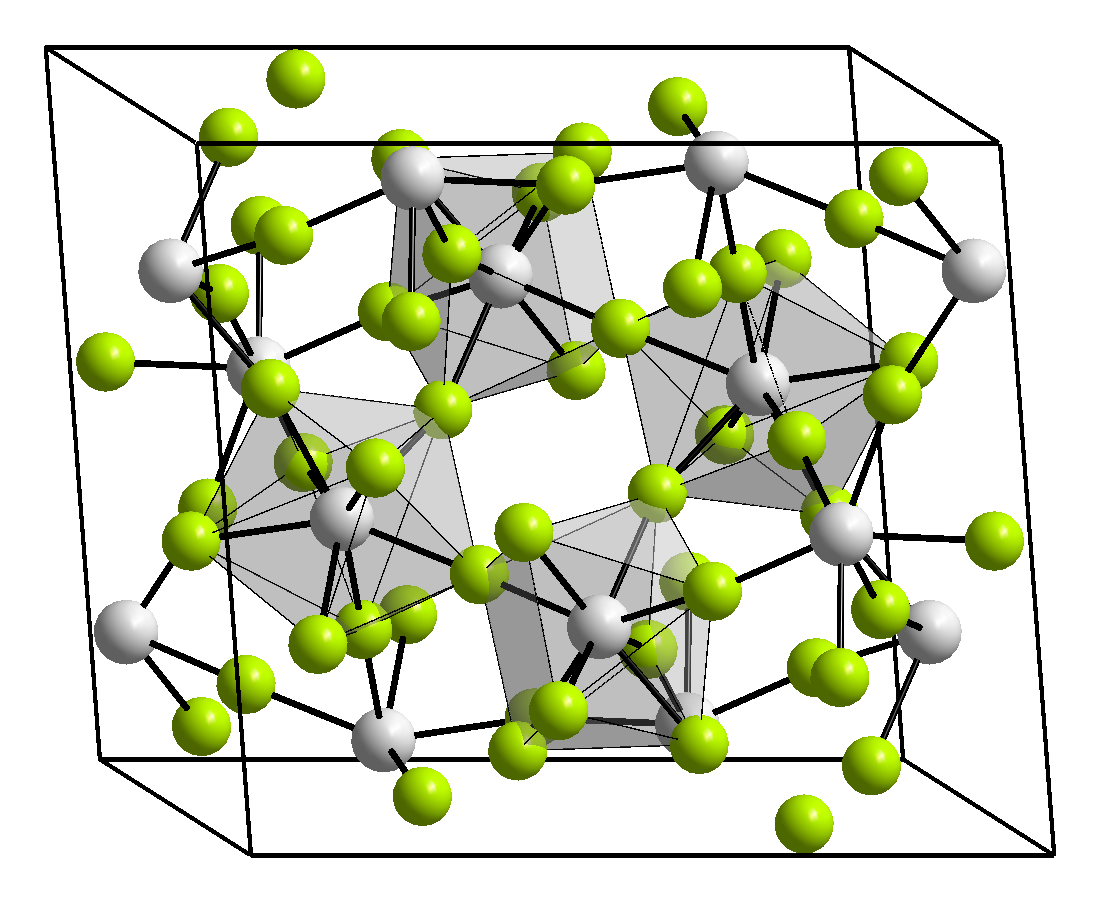
Structure

Three crystalline phases of ZrF4 have been reported, α (monoclinic), β (tetragonal, Pearson symbol tP40, space group P42/m, No 84) and γ (unknown structure). β and γ phases are unstable and irreversibly transform into the α phase at 400 °C.[3]
Zirconium(IV) fluoride forms several hydrates. The trihydrate has the structure (μ−F)2[ZrF3(H2O)3]2.[4]
Preparation and reactions
Zirconium fluoride can be produced by several methods. Zirconium dioxide reacts with hydrogen fluoride and hydrofluoric acid to afford the anhydrous and monohydrates:
- ZrO2 + 4 HF → ZrF4 + 2 H2O
The reaction of Zr metal reacts at high temperatures with HF as well:
- Zr + 4 HF → ZrF4 + 2 H2
Zirconium dioxide reacts at 200 °C with solid ammonium bifluoride to give the heptafluorozirconate salt, which can be converted to the tetrafluoride at 500 °C:
- 2ZrO2 + 7 (NH4)HF2 → 2 (NH4)3ZrF7 + 4 H2O + NH3
- (NH4)3ZrF7 → ZrF4 + 3 HF + 3 NH3
Addition of hydrofluoric acid to solutions of zirconium nitrate precipitates solid monohydrate. Hydrates of zirconium tetrafluoride can be dehydrated by heating under a stream of hydrogen fluoride.
Zirconium fluoride can be purified by distillation or sublimation.[2]
Zirconium fluoride forms double salts with other fluorides. The most prominent is potassium hexafluorozirconate, formed by fusion of potassium fluoride and zirconium tetrafluoride:[5]
- ZrF4 + 2 KF → K2ZrF6
Remove ads
Applications
The major and perhaps only commercial application of zirconium fluoride is as a precursor to ZBLAN glasses.[2]
Mixture of sodium fluoride, zirconium fluoride, and uranium tetrafluoride (53-41-6 mol.%) was used as a coolant in the Aircraft Reactor Experiment. A mixture of lithium fluoride, beryllium fluoride, zirconium fluoride, and uranium-233 tetrafluoride was used in the Molten-Salt Reactor Experiment. (Uranium-233 is used in the thorium fuel cycle reactors.)[citation needed]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads