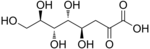
In organic chemistry, a sugar acid or acidic sugar is a monosaccharide with a carboxyl group at one end or both ends of its chain.[1]
Main classes of sugar acids include:
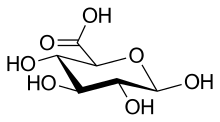
- Aldonic acids, in which the aldehyde group (−CH=O) located at the initial end (position 1) of an aldose is oxidized.
- Ulosonic acids, in which the hydroxymethyl group (−CH2OH) at the initial end of a 2-ketose is oxidized creating an α-ketoacid.
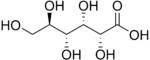
- Uronic acids, in which the −CH2OH group at the terminal end of an aldose or ketose is oxidized.
- Aldaric acids, in which both ends (−CH=O and −CH2OH) of an aldose are oxidized.
 |
 |
 |
Examples
Examples of sugar acids include:
- Aldonic acids
- Glyceric acid (3C)
- Xylonic acid (5C)
- Gluconic acid (6C)
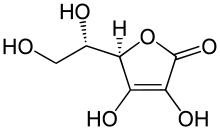
- Ascorbic acid[2] (6C, unsaturated lactone)
- Ulosonic acids
- Neuraminic acid (5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid)
- Ketodeoxyoctulosonic acid (KDO or 3-deoxy-D-manno-oct-2-ulosonic acid)
- Uronic acids
- Glucuronic acid (6C)
- Galacturonic acid (6C)
- Iduronic acid (6C)
- Aldaric acids
- Tartaric acid (4C)
- meso-Galactaric acid (Mucic acid) (6C)
- D-Glucaric acid (Saccharic acid) (6C)
 |
 |
References
External links
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.