Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
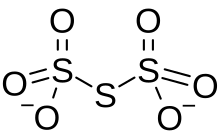
Trithionate is an oxyanion of sulfur with the chemical formula S
3O2−
6. It is the conjugate base of trithionic acid.[1] Dilute sodium hydroxide hydrolyzes S
4N
4 as follows, yielding sodium thiosulfate and sodium trithionate:
 | |
| Names | |
|---|---|
| IUPAC name
2,2,4,4-tetraoxido-1,5-dioxy-2,3,4-trisulfy-[5]catenate(2−) | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| 142337 | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| O6S3−2 | |
| Molar mass | 192.18 g·mol−1 |
| Conjugate acid | Trithionic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Certain sulfate-reducing bacteria have been known to use the compound in respiration.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.