Triethyl orthoformate
Chemical compound From Wikipedia, the free encyclopedia
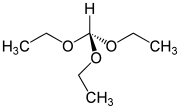
Triethyl orthoformate is an organic compound with the formula HC(OC2H5)3. This colorless volatile liquid, the ortho ester of formic acid, is commercially available. The industrial synthesis is from hydrogen cyanide and ethanol.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Diethoxymethoxy)ethane | |
| Other names
Triethoxymethane; Ethyl orthoformate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.138 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2524 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H16O3 | |
| Molar mass | 148.202 g·mol−1 |
| Density | 0.891 g/mL |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 146 °C (295 °F; 419 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Safety data sheet (SDS) | Fischer Scientific |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It may also be prepared from the reaction of sodium ethoxide, formed in-situ from sodium and absolute ethanol, and chloroform:[2]
- CHCl3 + 3 Na + 3 EtOH → HC(OEt)3 + 3⁄2 H2 + 3 NaCl
Triethyl orthoformate is used in the Bodroux-Chichibabin aldehyde synthesis, for example:[3]
- RMgBr + HC(OC2H5)3 → RC(H)(OC2H5)2 + MgBr(OC2H5)
- RC(H)(OC2H5)2 + H2O → RCHO + 2 C2H5OH
In coordination chemistry, it is used to convert metal aquo complexes to the corresponding ethanol complexes:[4]
- [Ni(H2O)6](BF4)2 + 6 HC(OC2H5)3 → [Ni(C2H5OH)6](BF4)2 + 6 HC(O)(OC2H5) + 6 HOC2H5
Triethyl orthoformate (TEOF) is an excellent reagent for converting compatible carboxylic acids to ethyl esters. Such carboxylic acids, refluxed neat in excess TEOF until low-boilers cease evolution, are quantitatively converted to the ethyl esters, without need for extraneous catalysis.[5] Alternatively, added to ordinary esterifications using catalytic acid and ethanol, TEOF helps drive esterification to completion by converting the byproduct water formed to ethanol and ethyl formate.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
