Tin(II) oxide
Chemical compound, stannous oxide (SnO) From Wikipedia, the free encyclopedia
Tin(II) oxide (stannous oxide) is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Tin(II) oxide | |
| Other names
Stannous oxide Tin monoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.040.439 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| SnO | |
| Molar mass | 134.709 g·mol−1 |
| Appearance | black or red powder when anhydrous, white when hydrated |
| Density | 6.45 g/cm3 |
| Melting point | 1,080 °C (1,980 °F; 1,350 K)[1] |
| insoluble | |
| −19.0·10−6 cm3/mol | |
| Structure | |
| tetragonal | |
| Thermochemistry | |
Std molar entropy (S⦵298) |
56 J·mol−1·K−1[2] |
Std enthalpy of formation (ΔfH⦵298) |
−285 kJ·mol−1[2] |
| Hazards | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[3] |
REL (Recommended) |
TWA 2 mg/m3[3] |
IDLH (Immediate danger) |
N.D.[3] |
| Safety data sheet (SDS) | ICSC 0956 |
| Related compounds | |
Other anions |
Tin sulfide Tin selenide Tin telluride |
Other cations |
Carbon monoxide Silicon monoxide Germanium(II) oxide Lead(II) oxide |
| Tin dioxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation and reactions
Summarize
Perspective

Blue-black SnO can be produced by heating the tin(II) oxide hydrate, SnO·xH2O (x < 1) precipitated when a tin(II) salt is reacted with an alkali hydroxide such as NaOH.[4]
Metastable, red SnO can be prepared by gentle heating of the precipitate produced by the action of aqueous ammonia on a tin(II) salt.[4]
SnO may be prepared as a pure substance in the laboratory, by controlled heating of tin(II) oxalate (stannous oxalate) in the absence of air or under a CO2 atmosphere. This method is also applied to the production of ferrous oxide and manganous oxide.[5][6]
- SnC2O4·2H2O → SnO + CO2 + CO + 2 H2O
Tin(II) oxide burns in air with a dim green flame to form SnO2.[4]
- 2 SnO + O2 → 2 SnO2
When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn3O4 which further reacts to give SnO2 and Sn metal.[4]
- 4SnO → Sn3O4 + Sn
- Sn3O4 → 2SnO2 + Sn
SnO is amphoteric, dissolving in strong acid to give tin(II) salts and in strong base to give stannites containing Sn(OH)3−.[4] It can be dissolved in strong acid solutions to give the ionic complexes Sn(OH2)32+ and Sn(OH)(OH2)2+, and in less acid solutions to give Sn3(OH)42+.[4] Note that anhydrous stannites, e.g. K2Sn2O3, K2SnO2 are also known.[7][8][9] SnO is a reducing agent and is thought to reduce copper(I) to metallic clusters in the manufacture of so-called "copper ruby glass".[10]
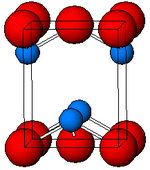
Structure
Black, α-SnO adopts the tetragonal PbO layer structure containing four coordinate square pyramidal tin atoms.[11] This form is found in nature as the rare mineral romarchite.[12] The asymmetry is usually simply ascribed to a sterically active lone pair; however, electron density calculations show that the asymmetry is caused by an antibonding interaction of the Sn(5s) and the O(2p) orbitals.[13] The electronic structure and chemistry of the lone pair determines most of the properties of the material.[14]
Non-stoichiometry has been observed in SnO.[15]
The electronic band gap has been measured between 2.5eV and 3eV.[16]
Uses
The dominant use of stannous oxide is as a precursor in manufacturing of other, typically divalent, tin compounds or salts. Stannous oxide may also be employed as a reducing agent and in the creation of ruby glass.[17] It has a minor use as an esterification catalyst.
Cerium(III) oxide in ceramic form, together with Tin(II) oxide (SnO) is used for illumination with UV light.[18]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
