Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
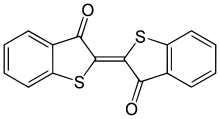
Thioindigo is an organosulfur compound that is used to dye polyester fabric. A synthetic dye, thioindigo is related to the plant-derived dye indigo, replacing two NH groups with two sulfur atoms to create a shade of pink.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
[2(2′)E]-3H,3′H-[2,2′-Bi-1-benzothiophenylidene]-3,3′-dione | |
| Other names
DyStar, C.I. Vat Red 41, C.I. 73 300 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.580 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H8O2S2 | |
| Molar mass | 296.36 g·mol−1 |
| Appearance | Red solid |
| Melting point | 280 °C (536 °F; 553 K) |
| Insoluble | |
| Solubility in ethanol, xylene | Soluble[vague] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thioindigo is generated by the alkylation of the sulfur in thiosalicylic acid with chloroacetic acid. The resulting thioether cyclizes to 2-hydroxythianaphthene, which is easily converted to thioindigo.[1] The related compound 4,7,4',7'-tetrachlorothioindigo, also a commercially important dye (Pigment Red 88), can be prepared by chlorination of thioindigo.
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.