Theacrine
Chemical compound From Wikipedia, the free encyclopedia
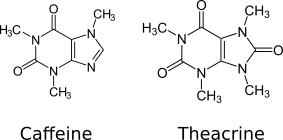
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu (Theobroma grandiflorum) and in a Chinese tea known as kucha (Chinese: 苦茶; pinyin: kǔ chá; lit. 'bitter tea') (Camellia assamica var. Kucha).[1][2] It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine.[2][3] In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.[2] Theacrine and caffeine are structurally similar.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,7,9-Tetramethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | |
| Other names
1,3,7,9-Tetramethyluric acid; Temurin; Temorine; Tetramethyluric acid; Tetramethyl uric acid; TeaCrine (trade name) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.268 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H12N4O3 | |
| Molar mass | 224.220 g·mol−1 |
| Melting point | 226 °C (439 °F; 499 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pharmacology
Pharmacodynamics
The exact mechanism of action of theacrine is uncertain, as no binding affinities have been published. However, animal research involving selective A1 and A2A adenosine agonists found theacrine pretreatment attenuated the expected motor depression induced by adenosine agonism, indicating that theacrine is likely an adenosine antagonist.[2]
Administration of selective dopamine D1 and D2 antagonists demonstrate that, similarly to caffeine,[4] the behavioural effects of theacrine are in part mediated by dopamine receptors.[2]
Pharmacokinetics
Safety
Theacrine has demonstrated clinical safety and non-habituating effects in healthy humans over eight weeks of daily use at up to 300 mg/day.[6] Moreover, there was no evidence of the tachyphylaxis typical of neuroactive agents like caffeine and other stimulants.[6]
In animal studies, theacrine has an LD50 of 810 mg/kg,[3][6] compared to 265 mg/kg for caffeine.[7]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.