Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
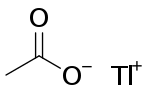
Thallous acetate or thallium(I) acetate is a salt of thallium and acetate with the chemical formula TlCH3COO. It is used in microbiology as a selective growth medium.[3] It is poisonous.[4]
 | |
| Names | |
|---|---|
| IUPAC name
Thallium(I) Acetate | |
| Other names
Thallium monoacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.416 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1707 3082 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| TlC2H3O2 | |
| Molar mass | 263.429 |
| soluble | |
| −69.0·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
35 mg/kg (mouse, oral) 41.3 mg/kg (rat, oral)[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.1 mg/m3 [skin][2] |
REL (Recommended) |
TWA 0.1 mg/m3 [skin][2] |
IDLH (Immediate danger) |
15 mg/m3 (as Tl)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.