Syringetin is an O-methylated flavonol, a type of flavonoid. It is found in red grape (absent in white grape),[1] in Lysimachia congestiflora[2] and in Vaccinium uliginosum (bog bilberries).[3] It is one of the phenolic compounds present in wine.[4]
Quick Facts Names, Identifiers ...
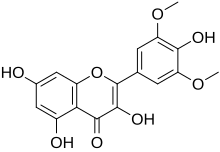
Syringetin
 |
| Names |
| IUPAC name
3,4′,5,7-Tetrahydroxy-3′,5′-dimethoxyflavone |
Systematic IUPAC name
3,5,7-Trihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-4H-1-benzopyran-4-one |
| Other names
3′,5′-O-Dimethylmyricetin
3′,5′-Dimethoxy-3,5,7,4′-tetrahydroxyflavone
3,5,7,4′-Tetrahydroxy-3′,5′-dimethoxyflavone |
| Identifiers |
|
|
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
|
|
| UNII |
|
|
|
InChI=1S/C17H14O8/c1-23-11-3-7(4-12(24-2)14(11)20)17-16(22)15(21)13-9(19)5-8(18)6-10(13)25-17/h3-6,18-20,22H,1-2H3  N NKey: UZMAPBJVXOGOFT-UHFFFAOYSA-N  N NInChI=1/C17H14O8/c1-23-11-3-7(4-12(24-2)14(11)20)17-16(22)15(21)13-9(19)5-8(18)6-10(13)25-17/h3-6,18-20,22H,1-2H3 Key: UZMAPBJVXOGOFT-UHFFFAOYAJ
|
COc1cc(cc(OC)c1O)C=3Oc2cc(O)cc(O)c2C(=O)C=3O
|
| Properties |
|
C17H14O8 |
| Molar mass |
346.291 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Close
It induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway.[4]
Syringetin is formed from laricitrin by the action of the enzyme laricitrin 5′-O-methyltransferase[1][5] (myricetin O-methyltransferase).[6]
Glycosides
- Syringetin-3-O-galactoside[1][7]
- Syringetin-3-O-glucoside[8][9]
- Syringetin 3-rhamnoside (CAS number 93126-00-2)
- Syringetin-3-O-rutinoside[9] found in Larix sibirica[10]
- Syringetin 3-O-(6′′-acetyl)-β-glucopyranoside found in Picea abies (Norway spruce)[11]
Mattivi, Fulvio; Guzzon, Raffaele; Vrhovsek, Urska; Stefanini, Marco; Velasco, Riccardo (2006). "Metabolite profiling of grape: Flavonols and anthocyanins". Journal of Agricultural and Food Chemistry. 54 (20): 7692–7702. doi:10.1021/jf061538c. PMID 17002441. S2CID 21407928. Lätti, Anja K.; Jaakola, Laura; Riihinen, Kaisu R.; Kainulainen, Pirjo S. (2010). "Anthocyanin and flavonol variation in bog bilberries (Vaccinium uliginosum L.) in Finland". Journal of Agricultural and Food Chemistry. 58 (1): 427–433. doi:10.1021/jf903033m. PMID 20000402. S2CID 28304488. Hsu, Ya-Ling; Liang, Hsin-Lin; Hung, Chih-Hsing; Kuo, Po-Lin (2009). "Syringetin, a flavonoid derivative in grape and wine, induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway". Molecular Nutrition & Food Research. 53 (11): 1452–1461. doi:10.1002/mnfr.200800483. PMID 19784998. S2CID 42240173. Tyukavkina, N. A.; Medvedeva, S. A.; Ivanova, S. Z. (1974). "New flavonol glycosides from the needles of Larix sibirica". Chemistry of Natural Compounds. 10 (2): 170–172. doi:10.1007/BF00563605. S2CID 4819832. Slimestad, Rune; Andersen, Øyvind M.; Francis, George W.; Marston, Andrew; Hostettmann, Kurt (1995). "Syringetin 3-O-(6′′-acetyl)-β-glucopyranoside and other flavonols from needles of Norway spruce, Picea abies". Phytochemistry. 40 (5): 1537–1542. doi:10.1016/0031-9422(95)00383-I. S2CID 84506810.
