Top Qs
Timeline
Chat
Perspective
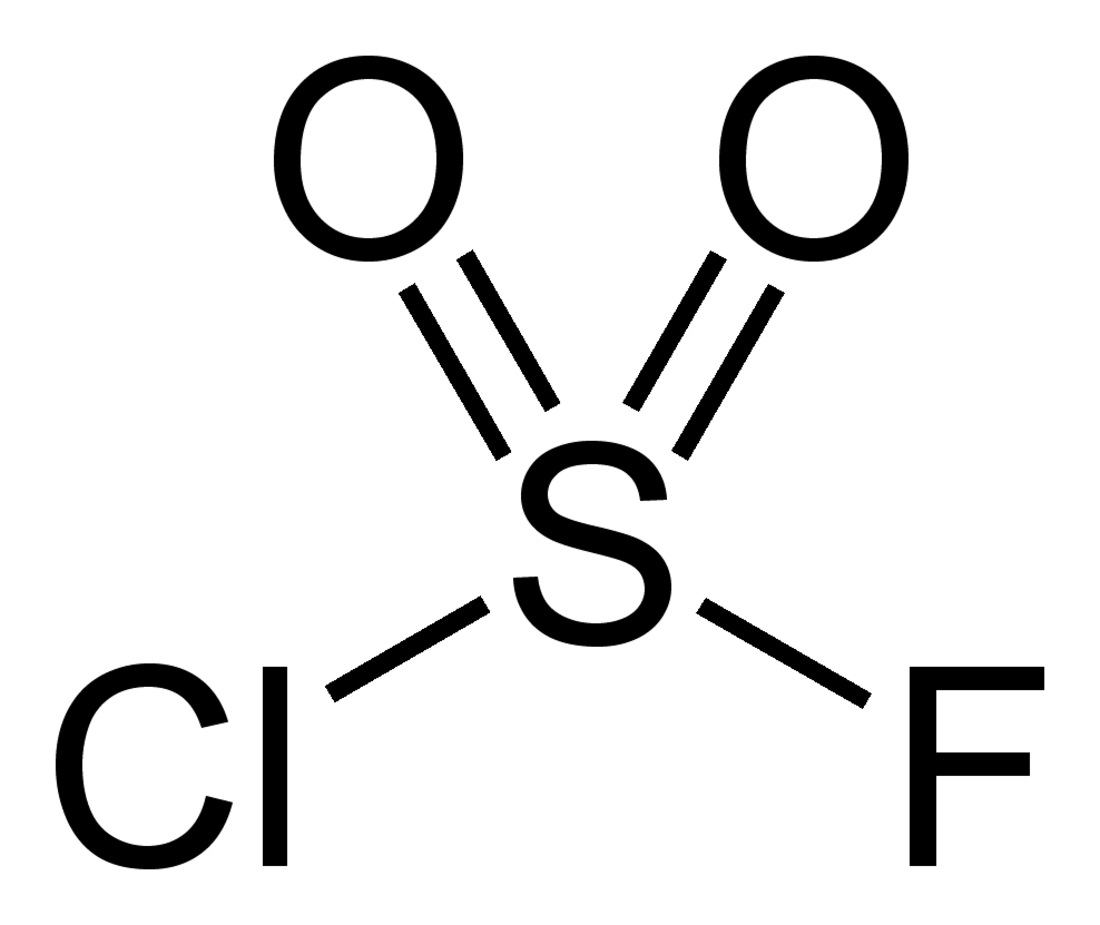
Sulfuryl chloride fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Sulfuryl chloride fluoride is a chemical compound with the formula SO2ClF. It is a colorless, easily condensed gas. It is a tetrahedral molecule.
Liquified sulfuryl chloride fluoride is employed as a solvent for highly oxidizing compounds.[1]
Remove ads
Preparation
The laboratory-scale synthesis begins with the preparation of potassium fluorosulfite:[2]
This salt is then chlorinated to give sulfuryl chloride fluoride[3]
Further heating (180 °C) of potassium fluorosulfite with the sulfuryl chloride fluoride gives sulfuryl fluoride.
- KSO2F + SO2ClF → SO2F2 + KCl + SO2
Alternatively, sulfuryl chloride fluoride can be prepared without using gases as starting materials by treating sulfuryl chloride with ammonium fluoride or potassium fluoride in trifluoroacetic acid.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads