Loading AI tools
SSRI drug and metabolite of fluoxetine From Wikipedia, the free encyclopedia
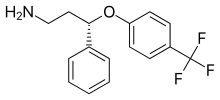
Seproxetine, also known as (S)-norfluoxetine, is a selective serotonin reuptake inhibitor (SSRI).[1][2] It is the S enantiomer of norfluoxetine, the main active metabolite of the widely used antidepressant fluoxetine;[3] it is nearly 4 times more selective for stimulating neurosteroid synthesis relative to serotonin reuptake inhibition than fluoxetine.[4] It is formed through the demethylation, or removal of a methyl group, of fluoxetine.[5] Seproxetine is both an inhibitor of serotonin and dopamine transporters, 5-HT2A and 5-HT2C receptors.[6] It was being investigated by Eli Lilly and Company as an antidepressant; however, it inhibited the KvLQT1 protein, which is responsible for the management of the QT interval. This is the time it takes for the heart to contract and recover. Due to the inhibition, the QT interval was prolonged, which could lead to significant cardiac side complications.[7] Due to this, development of the medication was discontinued.[1] Tests on its efficacy found that it was equivalent to fluoxetine, but sixteen times more powerful than the R enantiomer of norfluoxetine.[8]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 4–16 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H16F3NO |
| Molar mass | 295.305 g·mol−1 |
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.