Scandium phosphide
Chemical compound From Wikipedia, the free encyclopedia
Scandium phosphide is an inorganic compound of scandium and phosphorus with the chemical formula ScP.[3][4][5]
 | |
| Names | |
|---|---|
| Other names
Scandium monophosphide,[1] phosphanylidynescandium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.153 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| ScP | |
| Molar mass | 75.929670 g·mol−1 |
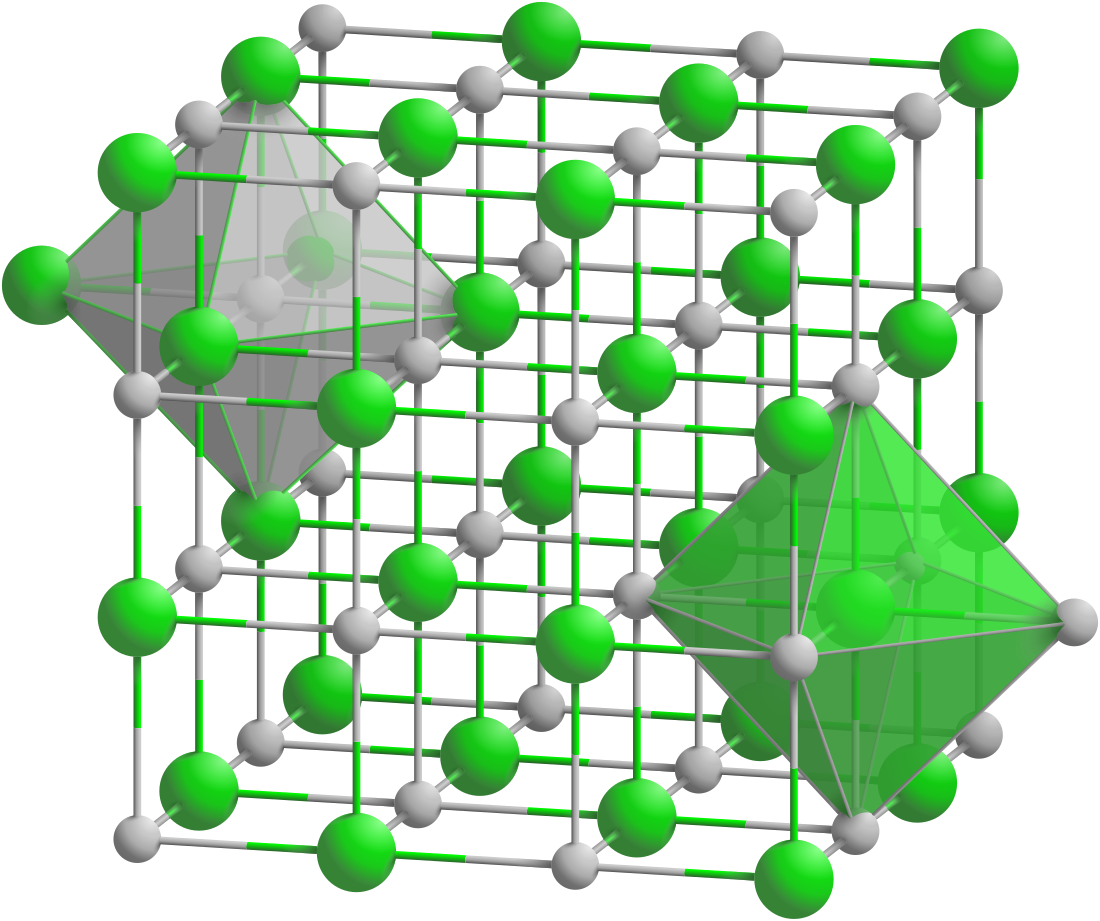
| Structure[2] | |
| Rock salt structure | |
| Fm3m | |
a = 0.5312 nm | |
Formula units (Z) |
4 |
| Octahedral at Sc3+, Octahedral at P3- | |
| Related compounds | |
Other anions |
|
Other cations |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
ScP can be obtained by the reaction of scandium and phosphorus at 1000 °C.[2]
- 4 Sc + P4 → 4 ScP
Physical properties
This compound is calculated to be a semiconductor used in high power, high frequency applications and in laser diodes.[6][7]
Chemical properties
ScP can be smelted with cobalt or nickel through electric arc to obtain ScCoP and ScNiP.[8]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.