Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal CO2 acceptor in plants.[1]: 2 It is a colourless anion, a double phosphate ester of the ketopentose (ketone-containing sugar with five carbon atoms) called ribulose. Salts of RuBP can be isolated, but its crucial biological function happens in solution.[2] RuBP occurs not only in plants but in all domains of life, including Archaea, Bacteria, and Eukarya.[3]
 The acid form of the RuBP anion | |
 | |
| Names | |
|---|---|
| IUPAC name
1,5-Di-O-phosphono-D-ribulose | |
| Other names
Ribulose 1,5-diphosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O11P2 | |
| Molar mass | 310.088 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
RuBP was originally discovered by Andrew Benson in 1951 while working in the lab of Melvin Calvin at UC Berkeley.[4][5] Calvin, who had been away from the lab at the time of discovery and was not listed as a co-author, controversially removed the full molecule name from the title of the initial paper, identifying it solely as "ribulose".[4][6] At the time, the molecule was known as ribulose diphosphate (RDP or RuDP) but the prefix di- was changed to bis- to emphasize the nonadjacency of the two phosphate groups.[4][5][7]
The enzyme ribulose-1,5-bisphosphate carboxylase-oxygenase (rubisco) catalyzes the reaction between RuBP and carbon dioxide. The product is the highly unstable six-carbon intermediate known as 3-keto-2-carboxyarabinitol 1,5-bisphosphate, or 2'-carboxy-3-keto-D-arabinitol 1,5-bisphosphate (CKABP).[8] This six-carbon β-ketoacid intermediate hydrates into another six-carbon intermediate in the form of a gem-diol.[9] This intermediate then cleaves into two molecules of 3-phosphoglycerate (3-PGA) which is used in a number of metabolic pathways and is converted into glucose.[10][11]
In the Calvin-Benson cycle, RuBP is a product of the phosphorylation of ribulose-5-phosphate (produced by glyceraldehyde 3-phosphate) by ATP.[11][12]
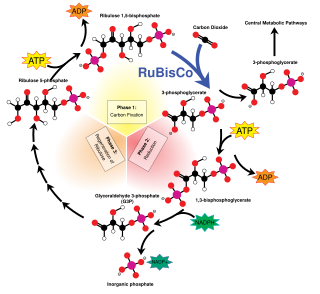
RuBP acts as an enzyme inhibitor for the enzyme rubisco, which regulates the net activity of carbon fixation.[13][14][15] When RuBP is bound to an active site of rubisco, the ability to activate via carbamylation with CO2 and Mg2+ is blocked. The functionality of rubisco activase involves removing RuBP and other inhibitory bonded molecules to re-enable carbamylation on the active site.[1]: 5
Rubisco also catalyzes RuBP with oxygen (O
2) in an interaction called photorespiration, a process that is more prevalent at high temperatures.[16][17] During photorespiration RuBP combines with O
2 to become 3-PGA and phosphoglycolic acid.[18][19][20] Like the Calvin-Benson Cycle, the photorespiratory pathway has been noted for its enzymatic inefficiency[19][20] although this characterization of the enzymatic kinetics of rubisco have been contested.[21] Due to enhanced RuBP carboxylation and decreased rubisco oxygenation stemming from the increased concentration of CO2 in the bundle sheath, rates of photorespiration are decreased in C4 plants.[1]: 103 Similarly, photorespiration is limited in CAM photosynthesis due to kinetic delays in enzyme activation, again stemming from the ratio of carbon dioxide to oxygen.[22]
RuBP can be measured isotopically via the conversion of 14CO2 and RuBP into glyceraldehyde 3-phosphate.[23] G3P can then be measured using an enzymatic optical assay.[23][24][lower-alpha 1] Given the abundance of RuBP in biological samples, an added difficulty is distinguishing particular reservoirs of the substrate, such as the RuBP internal to a chloroplast vs external. One approach to resolving this is by subtractive inference, or measuring the total RuBP of a system, removing a reservoir (e.g. by centrifugation), re-measuring the total RuBP, and using the difference to infer the concentration in the given repository.[25]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.