Top Qs
Timeline
Chat
Perspective
Pyroglutamic acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Pyroglutamic acid (also known as PCA, 5-oxoproline, pidolic acid) is a ubiquitous but understudied natural amino acid derivative in which the free amino group of glutamic acid or glutamine cyclizes to form a lactam.[1] The names of pyroglutamic acid conjugate base, anion, salts, and esters are pyroglutamate, 5-oxoprolinate, or pidolate.

It is a metabolite in the glutathione cycle that is converted to glutamate by 5-oxoprolinase. Pyroglutamate is found in many proteins including bacteriorhodopsin. N-terminal glutamic acid and glutamine residues can spontaneously cyclize to become pyroglutamate, or enzymatically converted by glutaminyl cyclases.[2] This is one of several forms of blocked N-termini which present a problem for N-terminal sequencing using Edman chemistry, which requires a free primary amino group not present in pyroglutamic acid. The enzyme pyroglutamate aminopeptidase can restore a free N-terminus by cleaving off the pyroglutamate residue.[3]
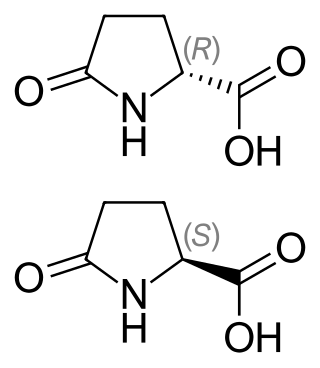
Pyroglutamic acid exists as two distinct enantiomers:
- (2R) or D which happens to be (+) or d
- (2S) or L which happens to be (–) or l
Remove ads
Metabolism
As first discovered in 1882, pyroglutamic acid can be formed by heating glutamic acid at 180 °C, which results in the loss of a molecule of water. In living cells, it is derived from glutathione through the action of an enzyme, γ-glutamyl cyclotransferase.[1] Pyroglutamic acid may function in glutamate storage, and acts to oppose the action of glutamate, including in the brain.[1] It also acts on the brain's cholinergic system;[4] Amyloid β containing pyroglutamic acid is increased in Alzheimer's disease; this may be part of the disease process.[5]
Increased levels of pyroglutamic acid in the blood, leading to excess in the urine (5-oxoprolinuria), can occur following paracetamol overdose, as well as in certain inborn errors of metabolism, causing high anion gap metabolic acidosis.[1][6]
Pyroglutamic acid is a natural humectant in skin,[7] and part of its natural moisturizing factor (NMF).[8]
Remove ads
Uses
The sodium salt of pyroglutamic acid—known either as sodium pyroglutamate, sodium PCA, or sodium pidolate—is used for dry skin and hair products, as it is a humectant. It has low toxicity and is not a skin irritant, but its use in products is limited by a high price.[9][10]
L-pyroglutamic acid is sold online as a nootropic dietary supplement.[11][12]
Magnesium pidolate, the magnesium salt of pyroglutamic acid, is found in some mineral supplements.
In a preclinical study, additional pharmacological properties of pyroglutamic acid were revealed such as anti-phosphodiesterase type 5, anti-angiotensin-converting enzyme, and anti-urease activities.[13]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads