Top Qs
Timeline
Chat
Perspective
Potassium polonide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium polonide is a chemical compound with the formula K2Po. It is a polonide, a set of very chemically stable compounds of polonium.[2][3]
Remove ads
Characteristics
Potassium polonide is thermally more unstable and has stronger electron affinity than potassium telluride (K2Te).[2][3]
Production
Potassium polonide may be produced from a redox reaction between polonium hydride and potassium metal:[2][3]
- H2Po + 2 K → K2Po + H2
It may also be produced by heating potassium and polonium together at 300–400 °C.[1] At higher temperature, this reaction may reverse.
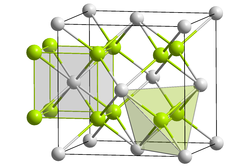
Crystal structure
Like sodium polonide, potassium polonide has the antifluorite structure.[2][3]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads