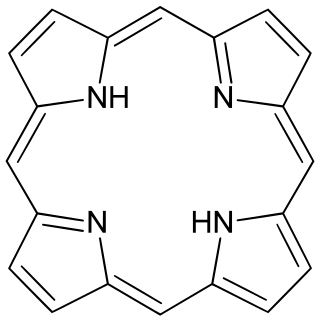
Porphine
Chemical compound From Wikipedia, the free encyclopedia
Porphine or porphin is an organic compound of empirical formula C20H14N4. It is heterocyclic and aromatic. The molecule is a flat macrocycle, consisting of four pyrrole-like rings joined by four methine bridges, which makes it the simplest of the tetrapyrroles.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Porphyrin[1] | |
| Other names
Porphin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.690 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H14N4 | |
| Molar mass | 310.35196 g/mol |
| Appearance | Dark red, shiny leaflets |
| Melting point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The nonpolar tetrapyrrolic ring structure of porphine means it is poorly soluble in most organic solvents and hardly water soluble.[3] As a result, porphine is mostly of theoretical interest. It has been detected in GC-MS of certain fractions of Piper betle.[4]
Porphine derivatives: porphyrins
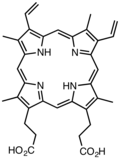
Substituted derivatives of porphine are called porphyrins. Many porphyrins are found in nature with the dominant example being protoporphyrin IX.[5] Many synthetic porphyrins are also known, including octaethylporphyrin[6] and tetraphenylporphyrin.[7]
- Common porphyrins
- Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.
- Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
- Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methine centers are occupied by phenyl groups.

Further reading
- Budavari, Susan (1989). "7574. Porphine". The Merck Index (11th ed.). Merck & Co., Inc. p. 1210. ISBN 0-911910-28-X. LCCN 89-60001.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.