Phenylacetone
Chemical compound From Wikipedia, the free encyclopedia
Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Phenylpropan-2-one | |
| Other names
Benzyl methyl ketone; Methyl benzyl ketone; Phenyl-2-propanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.859 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.178 g·mol−1 |
| Appearance | Colorless, pleasant odor |
| Density | 1.006 g/mL |
| Melting point | −15 °C (5 °F; 258 K) |
| Boiling point | 214 to 216 °C (417 to 421 °F; 487 to 489 K) |
| −83.44·10−6 cm3/mol | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known as P2P.[2][3] Due to illicit drug labs using phenylacetone to make amphetamines, phenylacetone was declared a schedule II controlled substance in the United States in 1980.[4] In humans, phenylacetone occurs as a metabolite of amphetamine and methamphetamine via FMO3-mediated oxidative deamination.[5]
Synthesis
There are many routes to synthesize phenylacetone. Industry uses the gas-phase ketonic decarboxylation of phenylacetic acid using acetic acid over a ceria-alumina solid acid catalyst.[6] A related laboratory-scale reaction has been described.[7]
An alternative route is zeolite-catalyzed isomerization of phenylpropylene oxide. Another laboratory synthesis involves conventional routes including the Friedel-Crafts alkylation reaction of chloroacetone with benzene in the presence of aluminum chloride catalyst.[8]
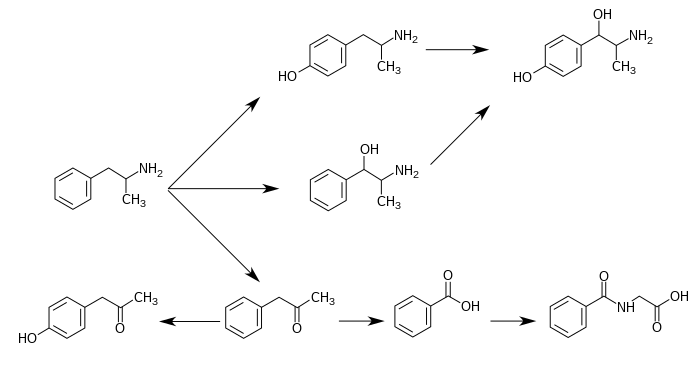
Amphetamine metabolism
Metabolic pathways of amphetamine in humans[sources 1]
|
Phenylacetone is an intermediate in the biodegradation of amphetamine. In the human liver, flavin-containing monooxygenase 3 (FMO3) deaminates amphetamines into phenylacetone, which is non-toxic to humans.[20] Phenylacetone is oxidized to benzoic acid, which is converted to hippuric acid by glycine N-acyltransferase (GLYAT) enzymes prior to excretion.
Phenylacetone can undergo para-hydroxylation to 4-hydroxyphenylacetone, which occurs as a metabolite of amphetamine in the human body.
Regulation and culture
To prevent illicit synthesis of amphetamines from phenylacetone, the precursor phenylacetic acid is subject to regulation in the United States under the Chemical Diversion and Trafficking Act.
In the TV series Breaking Bad, Walter White manufactures methamphetamine using phenylacetone and methylamine through a reductive amination reaction. White produced phenylacetone in a tube furnace using phenylacetic acid and acetic acid. [citation needed]

See also
- MDP2P – related compound with a methylenedioxy group, and a precursor to MDMA.
- Cyclohexylacetone – the cyclohexane derivative of phenylacetone
- Phenylacetones
- Methamphetamine
- Controlled Substances Act
Notes
- 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[10][15] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[15][17] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[18][19]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.