Nuclear pore glycoprotein p62
is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa[5] followed by modification that involve addition of N-acetylglucosamine residues,[6] followed by association with other complex proteins. In humans it is encoded by the NUP62 gene.
Quick Facts NUP62, Available structures ...
Close
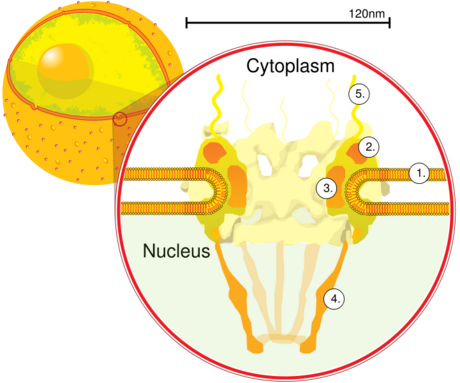
The nuclear pore complex is a massive structure that extends across the nuclear envelope, forming a gateway that regulates the flow of macromolecules between the nucleus and the cytoplasm. Nucleoporins are the main components of the nuclear pore complex in eukaryotic cells. The protein encoded by this gene is a member of the FG repeat containing nucleoporins and is localized to the nuclear pore central plug. This protein associates with the importin alpha/beta complex which is involved in the import of proteins containing nuclear localization signals. Multiple transcript variants of this gene encode a single protein isoform.[7]
P62 is a serine/threonine rich protein of ~520 amino acids, with tetrapeptide repeats on the amino terminus and a series of alpha-helical regions with hydrophobic heptad repeats[8] forming beta-propeller domain. P62 assembles into a complex containing 3 addition proteins, p60, p54 and p45 [9][10] forming the p62 complex of ~235 kDa. O-GlcNAcylation appears to be involved in the assembly and disassembly of p62 into higher order complexes, and a serine/threonine rich linker region between Ser270 to Thr294 appear to be regulatory.[11] The p62 complex is localized to both the nucleoplasmic and cytoplasmic sides of the pore complex and the relative diameter of p62 complex relative to the nuclear pore complex suggests it interacts in pore gating.[12]
P62 appears to interact with mRNA during transport out of the nucleus.[13] P62 also interacts with a nuclear transport factor (NTF2) protein that is involved in trafficking proteins between cytoplasm and nucleus.[14] Another protein, importin (beta) binds to the helical rod section of p62, which also
binds NTF2 suggesting the formation of a higher order gating complex.[15] Karyopherin beta2 (transportin), a riboprotein transporter also interacts with p62.[16] P62 also interacts with Nup93,[17] and when Nup98 is depleted p62 fails to assemble with nuclear pore complexes.[18] Mutant pores could not dock/transport proteins with nuclear localization signals or M9 import signals.
Antibodies to p62 complex are involved in one or more autoimmune diseases. P62 glycosylation is increased in diabetes[19] and may influence its association with other diseases. p62 is also more frequent in Stage IV primary biliary cirrhosis and is prognostic for severe disease.[20]
Nucleoporin 62 has been shown to interact with:
Lubas WA, Smith M, Starr CM, Hanover JA (1995). "Analysis of nuclear pore protein p62 glycosylation". Biochemistry. 34 (5): 1686–94. doi:10.1021/bi00005a025. PMID 7849028. Bullock TL, Clarkson WD, Kent HM, Stewart M (1996). "The 1.6 angstroms resolution crystal structure of nuclear transport factor 2 (NTF2)". J. Mol. Biol. 260 (3): 422–31. doi:10.1006/jmbi.1996.0411. PMID 8757804. Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M (1997). "Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import". J. Mol. Biol. 266 (4): 722–32. doi:10.1006/jmbi.1996.0801. PMID 9102465. Miyachi K, Hankins RW, Matsushima H, Kikuchi F, Inomata T, Horigome T, Shibata M, Onozuka Y, Ueno Y, Hashimoto E, Hayashi N, Shibuya A, Amaki S, Miyakawa H (2003). "Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study". J. Autoimmun. 20 (3): 247–54. doi:10.1016/S0896-8411(03)00033-7. PMID 12753810. Yoshima T, Yura T, Yanagi H (Nov 1997). "The trimerization domain of human heat shock factor 2 is able to interact with nucleoporin p62". Biochem. Biophys. Res. Commun. 240 (1): 228–33. doi:10.1006/bbrc.1997.7662. PMID 9367915. Gamper C, van Eyndhoven WG, Schweiger E, Mossbacher M, Koo B, Lederman S (2000). "TRAF-3 interacts with p62 nucleoporin, a component of the nuclear pore central plug that binds classical NLS-containing import complexes". Mol. Immunol. 37 (1–2): 73–84. doi:10.1016/S0161-5890(00)00015-8. PMID 10781837.
- Stoffler D, Fahrenkrog B, Aebi U (1999). "The nuclear pore complex: from molecular architecture to functional dynamics". Curr. Opin. Cell Biol. 11 (3): 391–401. doi:10.1016/S0955-0674(99)80055-6. PMID 10395558.
- Geetha T, Wooten MW (2002). "Structure and functional properties of the ubiquitin binding protein p62". FEBS Lett. 512 (1–3): 19–24. doi:10.1016/S0014-5793(02)02286-X. PMID 11852044. S2CID 22029085.
- Carmo-Fonseca M, Kern H, Hurt EC (1991). "Human nucleoporin p62 and the essential yeast nuclear pore protein NSP1 show sequence homology and a similar domain organization". Eur. J. Cell Biol. 55 (1): 17–30. PMID 1915414.
- Paschal BM, Gerace L (1995). "Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62". J. Cell Biol. 129 (4): 925–37. doi:10.1083/jcb.129.4.925. PMC 2120498. PMID 7744965.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Grote M, Kubitscheck U, Reichelt R, Peters R (1996). "Mapping of nucleoporins to the center of the nuclear pore complex by post-embedding immunogold electron microscopy". J. Cell Sci. 108 (9): 2963–72. doi:10.1242/jcs.108.9.2963. PMID 8537436.
- Guan T, Müller S, Klier G, Panté N, Blevitt JM, Haner M, Paschal B, Aebi U, Gerace L (1996). "Structural analysis of the p62 complex, an assembly of O-linked glycoproteins that localizes near the central gated channel of the nuclear pore complex". Mol. Biol. Cell. 6 (11): 1591–603. doi:10.1091/mbc.6.11.1591. PMC 301313. PMID 8589458.
- Park I, Chung J, Walsh CT, Yun Y, Strominger JL, Shin J (1996). "Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region". Proc. Natl. Acad. Sci. U.S.A. 92 (26): 12338–42. doi:10.1073/pnas.92.26.12338. PMC 40352. PMID 8618896.
- Joung I, Strominger JL, Shin J (1996). "Molecular cloning of a phosphotyrosine-independent ligand of the p56lck SH2 domain". Proc. Natl. Acad. Sci. U.S.A. 93 (12): 5991–5. Bibcode:1996PNAS...93.5991J. doi:10.1073/pnas.93.12.5991. PMC 39176. PMID 8650207.
- Vadlamudi RK, Joung I, Strominger JL, Shin J (1996). "p62, a phosphotyrosine-independent ligand of the SH2 domain of p56lck, belongs to a new class of ubiquitin-binding proteins". J. Biol. Chem. 271 (34): 20235–7. doi:10.1074/jbc.271.34.20235. PMID 8702753.
- Hu T, Guan T, Gerace L (1996). "Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins". J. Cell Biol. 134 (3): 589–601. doi:10.1083/jcb.134.3.589. PMC 2120945. PMID 8707840.
- Bullock TL, Clarkson WD, Kent HM, Stewart M (1996). "The 1.6 angstroms resolution crystal structure of nuclear transport factor 2 (NTF2)". J. Mol. Biol. 260 (3): 422–31. doi:10.1006/jmbi.1996.0411. PMID 8757804.
- Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M (1997). "Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import". J. Mol. Biol. 266 (4): 722–32. doi:10.1006/jmbi.1996.0801. PMID 9102465.
- Yaseen NR, Blobel G (1997). "Cloning and characterization of human karyopherin β3". Proc. Natl. Acad. Sci. U.S.A. 94 (9): 4451–6. Bibcode:1997PNAS...94.4451Y. doi:10.1073/pnas.94.9.4451. PMC 20743. PMID 9114010.
- Yoshima T, Yura T, Yanagi H (1997). "The trimerization domain of human heat shock factor 2 is able to interact with nucleoporin p62". Biochem. Biophys. Res. Commun. 240 (1): 228–33. doi:10.1006/bbrc.1997.7662. PMID 9367915.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Kehlenbach RH, Dickmanns A, Kehlenbach A, Guan T, Gerace L (1999). "A Role for RanBP1 in the Release of CRM1 from the Nuclear Pore Complex in a Terminal Step of Nuclear Export". J. Cell Biol. 145 (4): 645–57. doi:10.1083/jcb.145.4.645. PMC 2133185. PMID 10330396.
- Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J (1999). "The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation". EMBO J. 18 (11): 3044–53. doi:10.1093/emboj/18.11.3044. PMC 1171386. PMID 10356400.
- Rachubinski RA, Marcus SL, Capone JP (1999). "The p56(lck)-interacting protein p62 stimulates transcription via the SV40 enhancer". J. Biol. Chem. 274 (26): 18278–84. doi:10.1074/jbc.274.26.18278. PMID 10373430.