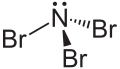Top Qs
Timeline
Chat
Perspective
Nitrogen tribromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Nitrogen tribromide is a chemical compound with the formula NBr3. It is extremely explosive in its pure form, even at −100 °C, and was not isolated until 1975.[2] It is a deep-red and volatile solid.
Remove ads
Preparation
NBr3 was first prepared by reaction of bistrimethylsilylbromamine (bis(trimethylsilyl)amine bromide) with bromine monochloride (with trimethylsilyl chloride as byproduct) at −87 °C according to the following equation:
- (Me3Si)2NBr + 2 BrCl → NBr3 + 2 Me
3SiCl
where "Me" is a methyl group.
NBr3 can be produced by the reaction of bromine or hypobromite and ammonia in a dilute aqueous buffer solution.[3] It can also be prepared by the reaction of bromine and bromine azide.[4] Ammonia and bromine undergo glow discharge, and after treatment, red NBr3·6NH3 can be obtained.[5] Pure nitrogen NBr3 was only produced in 1975.[6]
Remove ads
Reactions
Nitrogen tribromide reacts instantly with ammonia in dichloromethane solution at −87 °C to yield NBrH2.[7]
- NBr3 + 2 NH3 → 3 NH2Br
It also reacts with iodine in dichloromethane solution at −87 °C to produce NBr2I, which is a red-brown solid that stable up to -20 °C.[7]
- NBr3 + I2 → NBr2I + IBr
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads