Methylidynetricobaltnonacarbonyl
Chemical compound From Wikipedia, the free encyclopedia
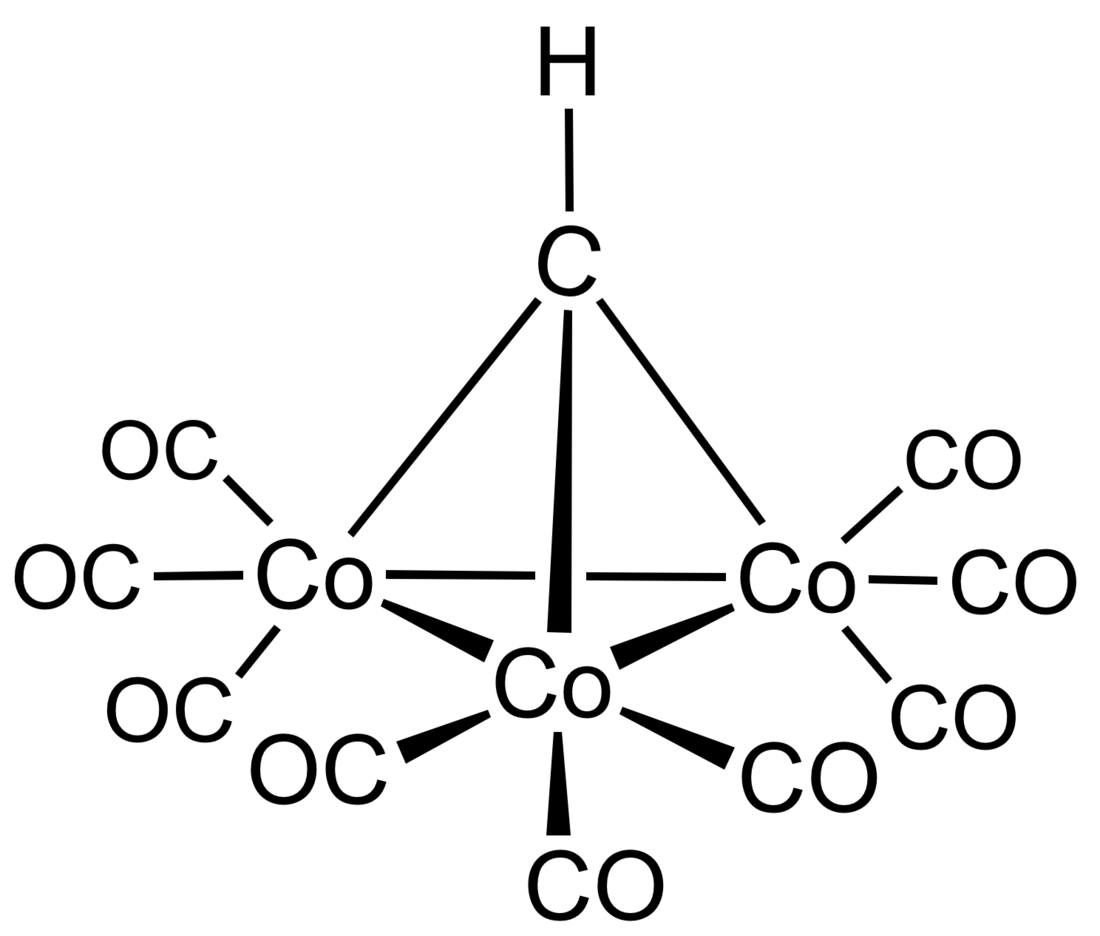
Methylidynetricobaltnonacarbonyl is an organometallic cobalt cluster with the chemical formula Co3(CO)9CH that contains a metal carbonyl core with the methylidyne ligand, first discovered in the late 1950s. A variety of substituents can be added to the methylidyne group to form derivatives of the parent compound that have unique spectroscopic properties and reactivity. This page will explore the discovery and synthesis of methylidynetricobaltnonacarbonyl, the structure and bonding of the parent compound, as well as some examples reactivity and catalysis with the cluster.
 | |
 | |
| Names | |
|---|---|
| Other names
methylidyne-tris(tricarbonylcobalt) | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C10HCo3O9 | |
| Molar mass | 441.909 g·mol−1 |
| Appearance | purple solid |
| Density | 2.01 g/cm3 |
| Melting point | 105–107 °C (221–225 °F; 378–380 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
Methylidynetricobaltnonacarbonyl and derivatives were discovered in the late 1950s by Markby and Wender by reactions of the alkyne complexes Co2(CO)6(R2C2) with acids. The structure of the cluster was however misformulated.[1] In 1962, the class of compounds were properly formulated methylidynetricobaltnonacarbonyl as well as several derivatives.[2]
The synthetic procedure developed by Bor and coworkers relied on the reaction of [Co(CO)4]− anion with chloroform, bromoform, or iodoform in a solution of acetone or THF. Dark violet crystals were obtained in ~20% yield with chloroform, ~18% yield with bromoform, and ~1-2% yield with iodoform. The chloride derivative Co3(CO)9CCl was obtained with CCl4 and was isolated as shiny, lilac crystals. Several other functional groups could be installed, including C6H5 and CO2CH3, all of which afforded dark violet crystals. Solutions of these compounds are air stable in organic solvents. Treatment of the parent compound with 100 atm CO at 160 °C resulted in the destruction of the complex and the formation of dicobaltoctacarbonyl.[2]
- 3NaCo(CO)4 + CHCl3 → HC[Co(CO)3]3 + 3 NaCl
Structure and Bonding
Summarize
Perspective
The Co-Co bond lengths are ~2.47 Å and the Co-C bond distances are ~1.88 Å.[2][3] These bond lengths do not change significantly depending on the substituent on the carbon group. The Co-C-Co angle is approximately 80°, indicating a significant bending of the carbon tetrahedral structure, and the ligands are arranged to provide maximum cobalt-carbon interaction.[4] The IR spectra show four absorption bands of varying intensities in the region of terminal C-O groups between 2111 and 2018 cm-1. The 13C NMR spectra have also been analyzed.[5] The mass spectra demonstrate parent molecular ions (Co3(CO)9CH .+) in good abundance, which demonstrates the high stability of the Co3(CO)9 clusters towards oxidation. Initial loss of CO, which is characteristic of most metal-carbonyls, though rupture of the metal-metal bond also often occurs.[4]

The carbonium ion had the correct symmetry to accept electron density from the apex carbon via s-p stabilization. They explained that apex carbon stabilize the carbonium ion via s-p conjugation.[7]

Reactivity
Summarize
Perspective
14CO labelling studies on the kinetics have been reported.[8] The group concluded the exchange rate of the carbonyl ligands decreases according to the order F > Cl > Br > H, which they attributed to the electronegativity of the atom bound to the methylidyne carbon. Additionally, they demonstrated that the nine carbonyl groups are not kinetically equivalent, and that only 3 CO groups exchange within the 35–55 °C temperature range studied, which agrees with the calculated frontier molecular orbitals discussed above. In the case with the COOCH3 group, the exchange occurs for only one CO group which they attributed to the more electron deficient nature of the complex.[8]
Methylidynetricobaltnonacarbonyls reacts with variety of organomercury compounds of the R2Hg and RHgX type; the side product Hg[Co(CO)4]2 was formed.[9]
Azobisisobutryonitrile (AIBN), a reagent for the initiation of radical reactions, to a solution of Co3(CO)9CH and allyl acetate afforded a red crystalline solid that they believed formed via a radical mechanism.[10] The radical reactivity of Co3(CO)9CX varies with X. The order of initiator activity was found to be X = Cl > H > Br > Ph > F > i-Pr > C2F5, which aligns with the trend seen for CO ligand exchange studies.[11][12] These cobalt carbonyl clusters undergo reversible, one-electron reduction in the range of -0.7 to -0.9V versus the saturated calomel electrode.[13]
Methylidynetricobaltnonacarbonyl catalyzes the Pauson-Khand reaction, a [2+2+1] cycloaddition.[14] Methylidynetricobaltnonacarbonyl is more air-stable than the parent dicobalt octacarbonyl, making it a more attractive catalyst. The clusters demonstrated no need for additives such as trimethylphosphite, as is necessary with the dicobalt octacarbonyl, and the best results were obtained with the parent methylidyne cluster.[14]

Methylidynetricobaltnonacarbonyl derivatives are precatalysts for the asymmetric intramolecular Pauson-Khand reaction. The chiral diphosphine Josiphos provides the asymmetry. Despite the unique approach, the clusters are inferior compared to other metal clusters with NORPHOS or Me-DuPHOS ligands, which gave higher yields and fewer side products.[15]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.