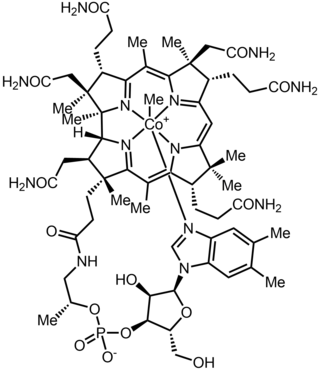
Methylcobalamin
Form of vitamin B12 From Wikipedia, the free encyclopedia
Methylcobalamin (mecobalamin, MeCbl, or MeB12) is a cobalamin, a form of vitamin B12. It differs from cyanocobalamin in that the cyano group at the cobalt is replaced with a methyl group.[1] Methylcobalamin features an octahedral cobalt(III) centre and can be obtained as bright red crystals.[2] From the perspective of coordination chemistry, methylcobalamin is notable as a rare example of a compound that contains metal–alkyl bonds. Nickel–methyl intermediates have been proposed for the final step of methanogenesis.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cobolmin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, sublingual, injection. |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.200 |
| Chemical and physical data | |
| Formula | C63H91CoN13O14P |
| Molar mass | 1344.405 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (what is this?) (verify) | |
Production

Methylcobalamin can be produced in the laboratory by reducing cyanocobalamin with sodium borohydride in alkaline solution, followed by the addition of methyl iodide.[2]
Functions
Summarize
Perspective
This vitamer, along with adenosylcobalamin, is one of two active coenzymes used by vitamin B12-dependent enzymes and is the specific vitamin B12 form used by 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), also known as methionine synthase.[citation needed]
Methylcobalamin participates in the Wood-Ljungdahl pathway, which is a pathway by which some organisms utilize carbon dioxide as their source of organic compounds. In this pathway, methylcobalamin provides the methyl group that couples to carbon monoxide (derived from CO2) to afford acetyl-CoA. Acetyl-CoA is a derivative of acetic acid that is converted to more complex molecules as required by the organism.[3]
Methylcobalamin is produced by some bacteria.[citation needed] It plays an important role in the environment, where it is responsible for the biomethylation of certain heavy metals. For example, the highly toxic methylmercury is produced by the action of methylcobalamin.[4] In this role, methylcobalamin serves as a source of "CH3+".
Role in human health
Methylcobalamin is equivalent physiologically to vitamin B12,[5][non-primary source needed] and can be used to prevent or treat pathology arising from a lack of vitamin B12 intake (vitamin B12 deficiency). Methylcobalamin is considered to be equivalent in efficacy to the other vitamin B12 vitamers as a dietary supplement, with no clear evidence of differing efficacy between them.[6][7][8]
Methylcobalamin that is ingested is not used directly as a cofactor, but is first converted by MMACHC into cob(II)alamin. Cob(II)alamin is then later converted into the other two forms, adenosylcobalamin and methylcobalamin for use as cofactors. That is, methylcobalamin is first dealkylated and then regenerated.[9][10][11]
Research directions
Ultra-high-dose intravenous methylcobalamin is researched as treatment of peripheral neuropathy, diabetic neuropathy, and as a preliminary treatment for amyotrophic lateral sclerosis.[12][13][14][15][16]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.