Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
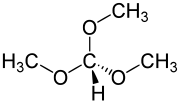
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers.[3] The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethoxymethane | |
| Other names
2-Methoxyacetaldehyde dimethyl acetal Methoxymethylal Methyl orthoformate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.224 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10O3 | |
| Molar mass | 106.121 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | pungent |
| Density | 0.9676 g/cm3 |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 100.6 °C (213.1 °F; 373.8 K) |
| Solubility | soluble in ethanol, diethyl ether |
| Vapor pressure | 1 kPa at 7 °C[2] |
Refractive index (nD) |
1.3773 |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 13 °C (55 °F; 286 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:[4]
Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis.
Trimethyl orthoformate is a useful building block for creating methoxymethylene groups and heterocyclic ring systems. It introduces a formyl group to a nucleophilic substrate, e.g. RNH2 to form R-NH-CHO, which can undergo further reactions. It is used in the production of some fungicides (for example: azoxystrobin and picoxystrobin), as well as for some members of the floxacin family of antibacterial drugs.
A number of pharmaceutical intermediates are also made from trimethyl orthoformate.[4]
Trimethyl orthoformate is also an effective reagent for converting compatible carboxylic acids to their corresponding methyl esters.[5] Alternatively, acid-catalyzed esterifications with methanol can be driven closer to completion by employing trimethyl orthoformate to convert water byproduct to methanol and methyl formate.
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.