Lithium selenide
Chemical compound From Wikipedia, the free encyclopedia
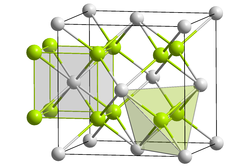 Crystal structure of lithium selenide __ Li+ __ Se2- | |
| Names | |
|---|---|
| IUPAC name
Lithium selenide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.015 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Li2Se | |
| Molar mass | 92.842 |
| Appearance | clear crystal[1] |
| Density | 2.0 g/cm3[2] |
| Melting point | 1,302 °C (2,376 °F; 1,575 K)[3] |
| hydrolysis[4] | |
| Structure | |
| cubic: anti-fluorite | |
| Fm3m, No. 225 | |
Formula units (Z) |
4 |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H261, H301, H331, H373, H410 | |
| P231+P232, P260, P261, P264, P270, P271, P273, P280, P301+P310, P304+P340, P311, P314, P321, P330, P370+P378, P391, P402+P404, P403+P233, P405, P501 | |
| Related compounds | |
Other anions |
Lithium oxide Lithium sulfide Lithium telluride Lithium polonide |
Other cations |
Sodium selenide Potassium selenide Rubidium selenide Caesium selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Properties
Lithium selenide is an inorganic compound that formed by selenium and lithium. It is a selenide with a chemical formula Li2Se. Lithium selenide has the same crystal form as other selenides, which is cubic, belonging to the anti-fluorite structure, the space group is , each unit cell has 4 units.[1]
Synthesis
Lithium Selenide can be synthesized via the reaction between 1.0 equivalents of grey elemental selenium and 2.1 equivalents of lithium trialkylborohydride. The reaction takes place in a solution of THF (tetrahydrofuran) under with stirring (minimum of 20 minutes) at room temperature according to the reaction below:[5] To increase yields and harmful byproducts, naphthalene can be added to the reaction as a catalyst.[6]
Se + 2Li(C2H5)3BH → Li2Se + 2(C2H5)3B + H2
Another method of synthesis involves the reduction of selenium with lithium in liquid ammonia. The Li2Se can be extracted after evaporation of the ammonia.[6]
Uses
One of the most contemporary uses of Li2Se compounds is in the creation of high-density capacitors and batteries. Lithium selenide can act as an excellent prelithiation agent, which helps to prevent the loss of capacity and efficiency during the formation of the solid electrolyte interphase (SEI). Additionally, the high relative conductivity and solubility of the products of lithium selenide decomposition makes it an ideal prelithiation agent. No harmful byproducts or gases are created during this decomposition of Li2Se.[7] One potential drawback to the use of Li2Se is the dissolution and shuttle problems inherent to the transition metals like selenide. To avoid this problem, evolving heterostructure materials can be used to inhibit the dissolution and shuttle effects of Li2Se.[8]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.

