Lanosterol
Chemical compound From Wikipedia, the free encyclopedia
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol.[1] In the eyes of vertebrates, lanosterol is a natural constituent, having a role in maintaining health of the lens. Lanosterol is the precursor to cholesterol.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lanosta-8,24-dien-3β-ol | |
| Systematic IUPAC name
(1R,3aR,5aR,7S,9aS,11aR)-3a,6,6,9a,11a-Pentamethyl-1-[(2R)-6-methylhept-5-en-2-yl]-2,3,3a,4,5,5a,6,7,8,9,9a,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol) |
|
| 2226449 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.105 |
| EC Number |
|
| KEGG | |
| MeSH | Lanosterol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.71 g/mol |
| Melting point | 138 to 140 °C (280 to 284 °F; 411 to 413 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Biosynthesis
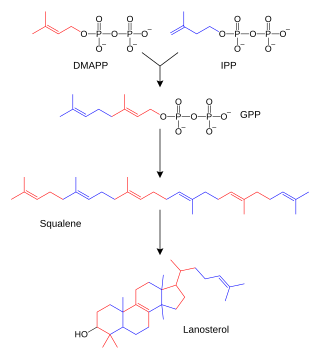
The biosynthesis of lanosterol has been intensively investigated.[2]
| Description | Illustration | Enzyme |
|---|---|---|
| Two molecules of farnesyl pyrophosphate condense with reduction by NADPH to form squalene | squalene synthase | |
| Squalene is oxidized to 2,3-oxidosqualene (squalene epoxide) |  | squalene monooxygenase |
| 2,3-Oxidosqualene is converted to a protosterol cation and finally to lanosterol |  | lanosterol synthase |
| (step 2) |  | (step 2) |
Elaboration of lanosterol under enzyme catalysis leads to other steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol.
Research as an eye drop supplement
As a molecule naturally enriched in the eye lens, lanosterol is a component involved in maintenance of lens clarity.[3][4] Its proposed mechanism of action is to inhibit the aggregation of crystallin proteins, which contribute to the clouding of vision by forming cataracts.[3][4]
Lanosterol is under research for its potential as a therapeutic additive in eye drops to inhibit the aggregation of crystallin proteins and dissolve cataracts.[3][4] However, supplemental lanosterol in eye drops appears to have limited solubility and poor bioavailability in the eye, and has not proved effective for inhibiting cataracts, as of 2020.[3][4]
See also
- Cycloartenol
- CYP51
- Other tetracyclic triterpenes: cycloartenol, euphol, tirucallol, and cucurbitacin.[2]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
