Iron(II) fluoride
Chemical compound From Wikipedia, the free encyclopedia
Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.[1][5]
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.232 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| FeF2 | |
| Molar mass | 93.84 g/mol (anhydrous) 165.902 g/mol (tetrahydrate) |
| Appearance | colorless transparent crystals[1] |
| Density | 4.09 g/cm3 (anhydrous) 2.20 g/cm3 (tetrahydrate) |
| Melting point | 970 °C (1,780 °F; 1,240 K) (anhydrous) 100 °C (tetrahydrate)[2] |
| Boiling point | 1,100 °C (2,010 °F; 1,370 K) (anhydrous) |
Solubility product (Ksp) |
2.36×10−6[3] |
| Solubility | insoluble in ethanol, ether; dissolves in HF |
| +9500.0·10−6 cm3/mol | |
| Structure | |
| Rutile (tetragonal), tP6 | |
| P42/mnm, No. 136 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Causes severe skin burns & eye damage; Hazardous decomposition products formed under fire conditions- Iron oxides[4] |
| GHS labelling: | |
   | |
| NFPA 704 (fire diamond) | |
| Flash point | not applicable[4] |
| Related compounds | |
Other anions |
Iron(II) chloride Iron(II) bromide Iron(II) iodide Iron(II) oxide |
Other cations |
Manganese(II) fluoride Cobalt(II) fluoride |
Related compounds |
Iron(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and bonding
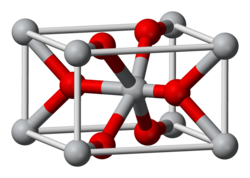
Anhydrous FeF2 adopts the TiO2 rutile structure. As such, the iron cations are octahedral and fluoride anions are trigonal planar.[6][7]
The tetrahydrate can exist in two structures, or polymorphs. One form is rhombohedral and the other is hexagonal, the former having a disorder.[1]
Like most fluoride compounds, the anhydrous and hydrated forms of iron(II) fluoride feature high spin metal center. Low temperature neutron diffraction studies show that the FeF2 is antiferromagnetic.[8] Heat capacity measurements reveal an event at 78.3 K corresponding to ordering of antiferromagnetic state.[9]
Selected physical properties
FeF2 sublimes between 958 and 1178 K. Using Torsion and Knudsen methods, the heat of sublimation was experimentally determined and averaged to be 271 ± 2 kJ mole−1.[10]
The following reaction is proposed in order to calculate the atomization energy for Fe+:[11]
- FeF2 + e → Fe+ + F2 (or 2F) + 2e
Synthesis and reactions
The anhydrous salt can be prepared by reaction of ferrous chloride with anhydrous hydrogen fluoride.[12] It is slightly soluble in water (with solubility product Ksp = 2.36×10−6 at 25 °C)[13] as well as dilute hydrofluoric acid, giving a pale green solution.[1] It is insoluble in organic solvents.[5]
The tetrahydrate can be prepared by dissolving iron in warm hydrated hydrofluoric acid and precipitating the result by addition of ethanol.[1] It oxidizes in moist air to give, inter alia, a hydrate of iron(III) fluoride, (FeF3)2·9H2O.[1]
Uses
Battery research
FeF2 has been investigated as a cathode material for both lithium-ion and fluoride-ion batteries. Unlike conventional metal oxides, which rely on an intercalation-based lithium storage mechanism, FeFX (x = 2, 3) operates via a complex conversion mechanism, resulting in higher energy density. Fluoride cathodes are stable up to 1000°C.[15] Stability not only enhances safety and lowers the risk of thermal runaway.[16]
FeFX exhibits distinctive phase evolution, intermediate phases, and morphological transformations during lithiation and delithiation.[17][18] A stable lattice of fluoride anions is maintained throughout charge and discharge cycles, consistent with high cycling reversibility.[19][20]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.

