Iodite
Ion From Wikipedia, the free encyclopedia
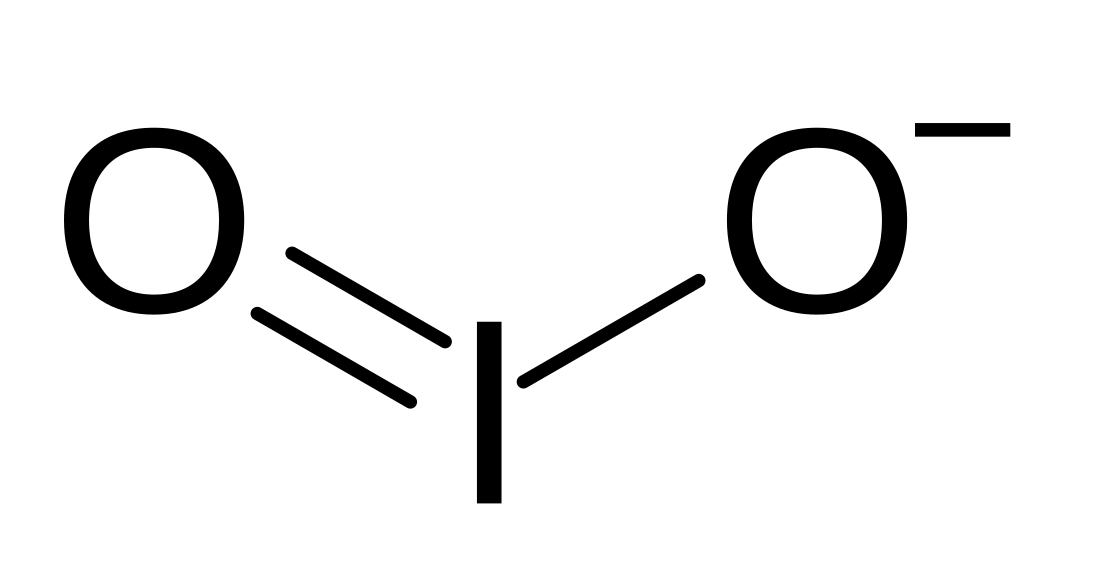
The iodite ion, or iodine dioxide anion, is the halite with the chemical formula IO−
2. Within the ion, the iodine exists in the oxidation state of +3.
 | |
| Names | |
|---|---|
| IUPAC name
iodite | |
| Systematic IUPAC name
dioxidoiodate(1−) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| IO− 2 | |
| Molar mass | 58.90 g/mol |
| Conjugate acid | Iodous acid |
| Related compounds | |
Other anions |
Chlorite Bromite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iodite anion
Iodites (including iodous acid) are highly unstable and have been observed[1] but never isolated.[citation needed] They will rapidly disproportionate to molecular iodine and iodates.[2] However, they have been detected as intermediates in the conversion between iodide and iodate.[3][4]
Other oxyanions
Iodine can assume oxidation states of −1, +1, +3, +5, or +7. A number of neutral iodine oxides are also known.
| Iodine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | iodide | hypoiodite | iodite | iodate | periodate |
| Formula | I− | IO− | IO− 2 |
IO− 3 |
IO− 4 or IO5− 6 |
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.