Top Qs
Timeline
Chat
Perspective
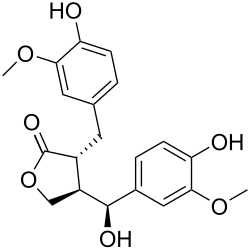
Hydroxymatairesinol
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hydroxymatairesinol (HMR) is a lignan found in Norway spruce (Picea abies).[1] It is an enterolactone precursor with anticancer activities. In rats, HMR decreased the volume of induced tumours and stabilised established tumours, as well as preventing the development of new tumours.[1] It has also shown anti-oxidant properties in vitro.[1]
HMR's chemical structure is similar to matairesinol.[2] At high concentrations, HMR has estrogenic properties, which are considerably weaker than those of estradiol.[2]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads